- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
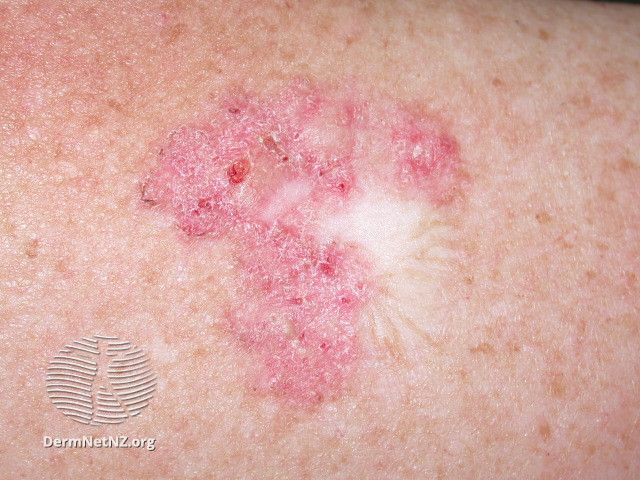
Last Patient Dosed in Verrica's Phase 2 Study of VP-315 for Basal Cell Carcinoma
Author(s):
The last patient has been dosed in the trial of the potential first-in-class oncolytic peptide for BCC.
Verrica Pharmaceuticals recently announced the last patient has been dosed in its phase 2 study of VP-315, a potential first-in-class oncolytic peptide-based immunotherapy, for the treatment of basal cell carcinoma (BCC).
The phase 2 trial (NCT05188729), which is a proof-of-concept, dose-escalation, multicenter, open-label, and 2-part study examining the efficacy, safety, and pharmacokinetics of the therapy in this indication.
In total, an expected 80 adult patients with a histological BCC diagnosis are expected to be enrolled in the trial. Each patient is required to have such a diagnosis in at least 1 target lesion.
VP-315 is administered into a tumor in order to induce immunogenic cell death. This, Verrica said, may offer a non-surgical option for patients with BCC.
“We are pleased to announce that Part 2 of our Phase 2 clinical trial of VP-315 for the treatment of basal cell carcinoma has been fully enrolled and the last patient has been dosed,” said Ted White, president and chief executive officer of Verrica, in a press release. “Basal cell carcinoma is the most common form of skin cancer in the US each year and patients are in need of alternative solutions to surgery which can cause pain, infection and scarring. Verrica’s VP-315 program is designed to provide for the targeted delivery of an oncolytic peptide engineered to stimulate the patient’s immune system and destroy cancer cells. Our study remains on track, and we look forward to sharing the data from our Phase 2 clinical trial later this year.”
Reference
BioSpace. Verrica Pharmaceuticals announces last patient dosed in part 2 of phase 2 study of VP-315, a potential first-in-class oncolytic peptide-based immunotherapy, for the treatment of basal cell carcinoma. BioSpace. January 5, 2024. Accessed January 5, 2024. https://www.biospace.com/article/releases/verrica-pharmaceuticals-announces-last-patient-dosed-in-part-2-of-phase-2-study-of-vp-315-a-potential-first-in-class-oncolytic-peptide-based-immunotherapy-for-the-treatment-of-basal-cell-carcinoma/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.