- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Leishmaniasis comes to the U.S.
Dermatologists practicing in the States may not routinely see leishmaniasis cases, but with global travel so common today, such tropical diseases may hitch a ride with patients into dermatologic practices throughout the U.S.
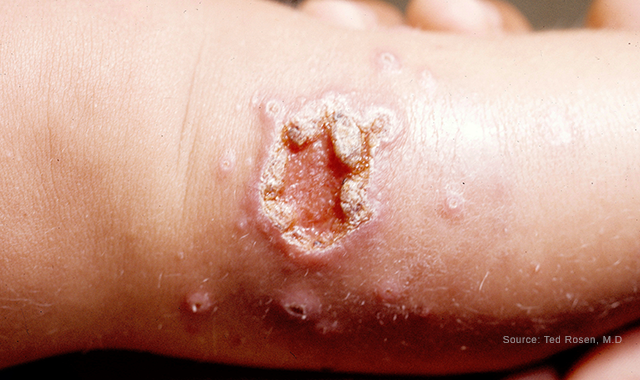
Classic ulcerative leishmaniasis on the leg of a child

Dermatologists practicing in the States may not routinely see leishmaniasis cases, but with global travel so common today, such tropical diseases may hitch a ride with patients into dermatologic practices throughout the U.S.
At Maui Derm 2015, Ted Rosen, M.D., professor of dermatology at Baylor College of Medicine and chief of dermatology at the Houston VA Medical Center, and Neal Bhatia M.D., director of clinical dermatology, Therapeutics Clinical Research in San Diego, discussed treatment approaches, including the recently approved miltefosine (Impavido), in their presentation, “New Drugs and New Concepts.”
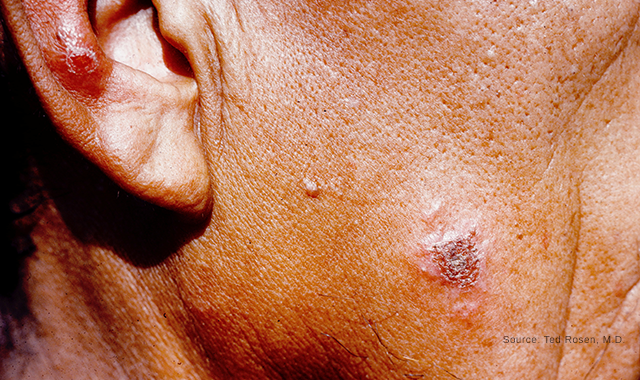
Early leishmaniasis lesions on the cheek and ear


Parasitic membrane
Dr. Rosen explains that although miltefosine-an oral medication that interferes with parasitic membrane protein kinase signaling-has been in use internationally for many years, it was approved for use in the U.S. in March, 2014. He notes that miltefosine is approximately 90% effective for L. braziliensis and related new world leishmaniasis types, and approximately 58% effective for L. mexicana. There is no solid data to predict efficacy against old world leishmania.
READ: Ancient medicine, modern MRSA cure?
“Since results are equal to antimony,” Dr. Rosen says, “but the drug is less toxic and easier to administer, it may become initial treatment of choice. However, it is still advisable to determine the precise species causing the disease, which in turn, predicts responsiveness to all potential interventions.” He notes that the CDC laboratory staff provides species determination, free of charge.1 “On the CDC website,2 one can find a guide to diagnosis of leishmaniasis,” Dr. Rosen explains. “This guide discusses the various types of specimens which can be submitted and how to submit them.”
Impavido is supplied in 50 mg capsules and dosage is titrated based on the patient’s weight; the treatment course is 28 days. The medication carries a pregnancy category X black box warning. Adequate contraception is mandatory during use and for five months after discontinuance, and the medication should not be administered to lactating women. Otherwise, adverse effects may include anorexia, nausea, vomiting, diarrhea, headache, and mild thrombocytopenia. “It can cause mild liver and renal toxicity,” says Dr. Rosen. “Common sense suggests caution in prescribing to patients with pre-existing liver or kidney disease.”
Course of treatment
How soon into the 28-day treatment regimen might one expect to see results?
“The rationale for most of these approaches is to treat through the disease, not just treat to the point where you see what looks clinically clear,” says Dr. Bhatia. “Looking at that, if there’s not a benefit to treating through the disease, the smart course of action would be at least to follow the patient for another month or two and observe for any response.”
Dr. Rosen concurs. “There is no indication for extension of treatment, as this has not been studied systematically. Clearance rates are too variable to generalize, but total clearance may require one or more months after the standard 28 day course is complete.”
Although miltefosine’s approval is good news, Dr. Rosen is pragmatic. “The first line of therapy remains pentavalent antimony. Thermotherapy is second line. This new oral therapy may supplant antimony as first line. However, there is considerable variability in response from country to country and even with the same species from different parts of the same country. Therefore, we will need to have multiple modalities available in case whatever is done first fails. Also, as of this writing, physically securing the drug for patients involves a rather circuitous route, including the prescriber obtaining an emergency investigational new drug number, sending the prescription to the manufacturer, and pre-paying for the drug [which is shipped from Europe].”
References:
1. CDC laboratory: 404-718-4175 or at DPDx@cdc.gov.
2. http://www.cdc.gov/parasites/leishmaniasis/health_professionals/index.html#dx





