- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Lentigo maligna: Topical agent option for malignant melanoma
Preliminary, yet promising, outcomes in two patients suggests dual therapy involving limited surgical excision followed by topical application of imiquimod 5 percent cream (Aldara, Graceway) may be an option to consider for treating invasive malignant melanoma surrounded by lentigo maligna (LM) in patients who refuse or are not good candidates for more extensive surgery, according to researchers from the department of dermatology, Saint Louis University School of Medicine, St. Louis.

Key Points
San Francisco - Preliminary, yet promising, outcomes in two patients suggests dual therapy involving limited surgical excision followed by topical application of imiquimod 5 percent cream (Aldara, Graceway) may be an option to consider for treating invasive malignant melanoma surrounded by lentigo maligna (LM) in patients who refuse or are not good candidates for more extensive surgery, according to researchers from the department of dermatology, Saint Louis University School of Medicine, St. Louis.

STUDY DETAILS
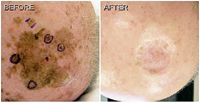
"Although the collective number of patients treated is limited, as is the duration of follow-up, this experience encouraged us to consider imiquimod in our patients with LM, including a focal invasive component, who may not opt for definitive surgery.
"To our knowledge, ours is the first report of such cases," Dr. Missall tells Dermatology Times.
"Data from more patients and from longer-term follow-up are needed to ultimately determine the role of topical imiquimod in the management of LM with and without an invasive component."
One of the patients treated with the dual therapy approach had one scalp lesion measuring nearly 8 cm by 8 cm, along with multiple other smaller scalp lesions. Multiple punch biopsies were obtained from the large lesion and revealed lentigo maligna melanoma (LMM) (Breslow depth 0.4 mm) at one site, while other specimens showed either LM or benign solar lentigo.
The invasive component was excised to the periosteum with 1 cm margins, and the defect was closed with a graft.
Topical imiquimod was started four weeks postsurgery, with the patient instructed to apply the cream to the entire lesion plus a 2 cm margin of normal-appearing skin.
Treatment was initiated five times a week and was continued for 14 weeks, although for four weeks, the frequency of application was reduced to three times a week to allow the patient to tolerate an intense inflammatory response.





