- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
At long lash: Cosmetic derms welcome Latisse; still, others worry
National report - A new prescription product that promotes eyelash growth (Latisse, bimatoprost ophthalmic solution 0.03 percent; Allergan) will benefit cosmetic-minded patients and the dermatologists who serve them, sources say.

Key Points
National report - A new prescription product that promotes eyelash growth (Latisse, bimatoprost ophthalmic solution 0.03 percent; Allergan) will benefit cosmetic-minded patients and the dermatologists who serve them, sources say.
However, some dermatologists worry about the product's potential side effects and the quality of the research behind it.
In late December, the Food and Drug Administration (FDA) approved Latisse for treating eyelash hypotrichosis.
In a phase 3 study, investigators enrolled 278 adults with minimal or moderate eyelash prominence, randomizing them to apply Latisse or a placebo to both upper eyelid margins once nightly for 16 weeks.
Lash: What are the possible side effects of Latisse?
Lash: What are the possible side effects of Latisse?
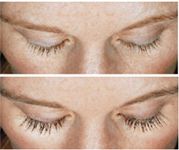
Additionally, Latisse achieved more than 100 percent mean improvement over baseline for eyelash thickness. Patients tolerated Latisse well, Dr. Fagien says.
However, he says, "It's important for patients to understand that they may experience redness for the first few days and maybe weeks of treatment. But in the majority of cases, over time, the redness decreases or disappears."
Patients also must understand that applying more bimatoprost than prescribed won't necessarily boost efficacy, Dr. Fagien says. But doing so may increase incidence of unwanted side effects, such as lid-margin irritation, Steven Yoelin, M.D., a Newport Beach, Calif., ophthalmologist in private practice, tells Dermatology Times.
Dr. Fagien adds that in the phase 3 study, Latisse did not significantly lower intraocular pressure. And although Latisse's label warns of possible changes in iris pigmentation, he says that because Latisse uses only 5 percent of the dose required for treating glaucoma, investigators observed no such changes.
Long-term effects?
Nevertheless, Amy E. Newburger, M.D., says, "I'm not keen about the side effects. Investigators did report that a small number of study patients developed discoloration of the eyelid skin," which in the context of glaucoma treatments has proven very tough to treat.
Furthermore, she says Latisse conceivably could lower intraocular pressure, "and I expect it will. This could conceal the diagnosis of glaucoma."
And a four-month trial won't show whether the product changes iris color because this effect doesn't occur until at least seven months' use, says Dr. Newburger, who is director, Dermatology Consultants of Westchester, N.Y.
Therefore, she says that although she is besieged by requests for Latisse, "I'll wait to see what happens with large numbers of patients before I decide whether to endorse it."
For now, she says she'll likely prescribe Latisse only if patients get an ophthalmologist's clearance, or send results of an eye exam conducted within the previous year.
Overall, she says, "I'm cautious, and I'm pleased that Allergan tested it" using the FDA drug-approval path.





