- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Merger to boost eczema offerings
Author(s):
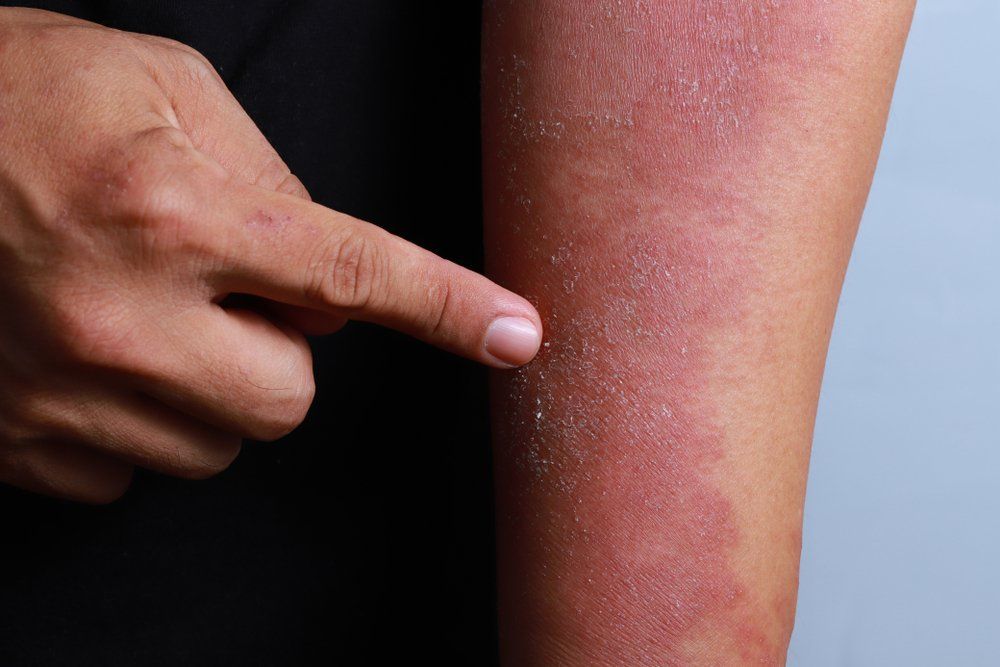
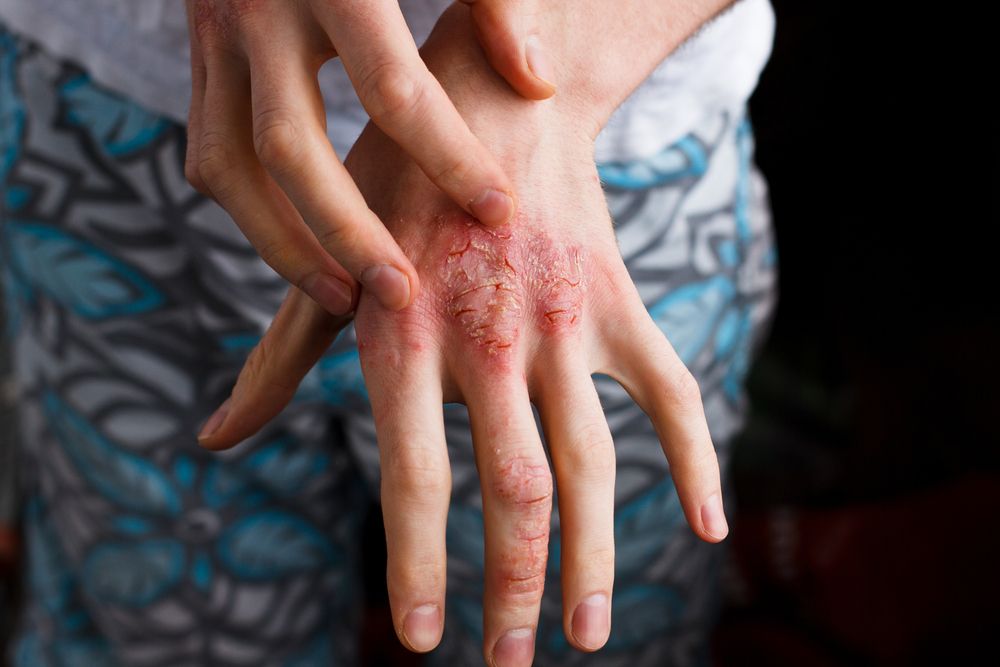
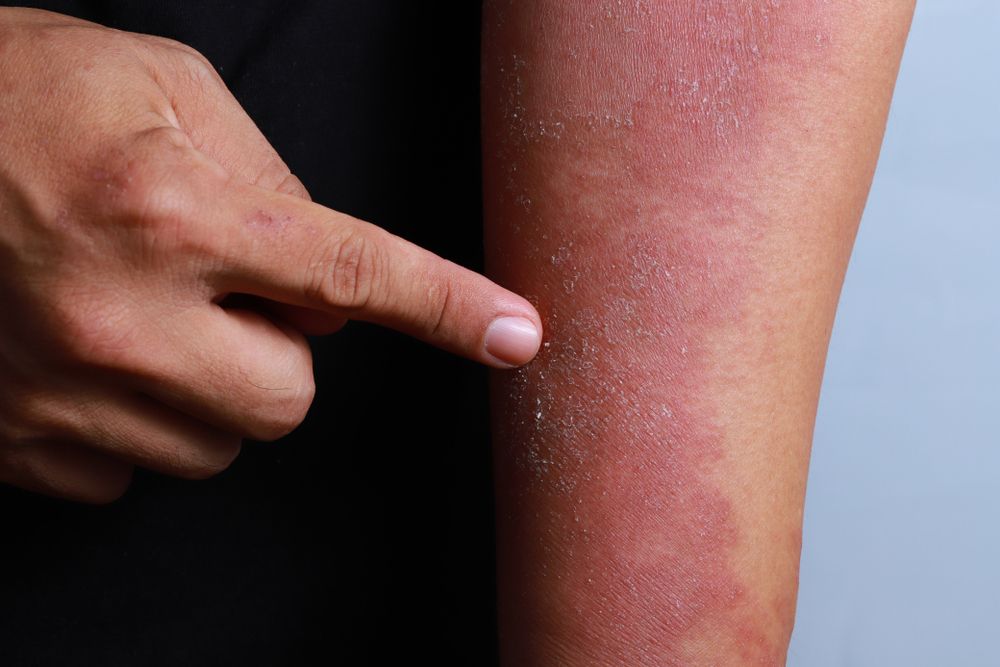
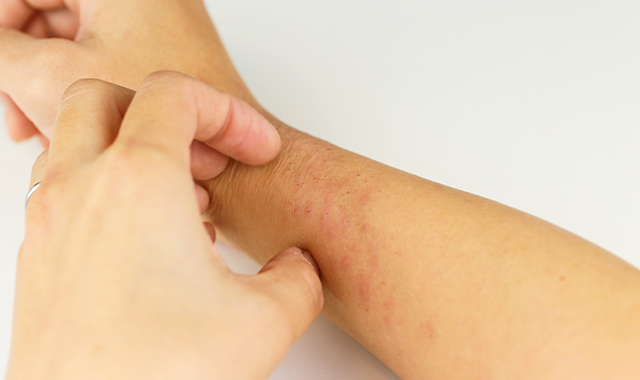
Pfizer and Anacor Pharmaceuticals announced in May that the pharmaceutical companies merged with Pfizer acquiring Anacor for about $5.2 billion. Dermatologists say merger is a good move for patients.
Pfizer and Anacor Pharmaceuticals announced in May that the pharmaceutical companies merged with Pfizer acquiring Anacor for about $5.2 billion.
The FDA is reviewing Anacor’s flagship asset crisaborole, topical ointment, 2%, a novel non-steroidal topical anti-inflammatory PDE-4 inhibitor, for treatment of mild-to-moderate atopic dermatitis. With a potential approval and launch in early to mid-2017, crisaborole could be a first-line treatment option for eczema patients, and Pfizer predicts a peak sales year for the drug could be $2 billion or more.
While the Pfizer deal will most likely fuel crisaborole’s market potential, crisaborole’s approval could pave the way for Pfizer to launch its eczema pipeline candidate, a topical form of tofacitinib (Xeljanz), which completed a phase 2a trial in atopic dermatitis in September 2015.
If approved, crisaborole could be the first to enter what has been a dormant mild-to-moderate eczema treatment marketplace. Abhilok Garg, Ph.D., GlobalData’s analyst covering immunology, says in a press release: “Crisaborole … has set itself apart from the competition with significant investment into proving its safety in pediatric patients, a largely underserved demographic.”

Dr. SiegfriedDermatologists we asked about the merger say they think it’s a good move for patients.
“Atopic dermatitis is a common, chronic condition with limited therapeutic options for long-term control,” says Elaine C. Siegfried, M.D., professor of pediatrics and dermatology, Saint Louis University, St. Louis, Mo. “I am pleased that the pharmaceutical industry is finally focusing attention and resources on developing alternative treatments, and including children in the process.”
The Pfizer-Anacor merger is an exciting change in the dermatology market, according to Lawrence F. Eichenfield, M.D., professor of dermatology and pediatrics and chief of pediatric dermatology, University of California, San Diego and Rady Children's Hospital, San Diego.

Dr. Eichenfeld“There have been no new chemical entities for the topical treatment of atopic dermatitis in almost 15 years, and patients will be interested in crisaborole, a non-steroid agent with studies showing good safety,” Dr. Eichenfield says. “Pfizer has made a big investment in dermatology, and they may be gearing up to develop new systemic, as well as new topical medications for dermatitis as well as other inflammatory skin diseases.”
Disclosure: Dr. Eichenfield has served as a consultant for Anacor and Pfizer, but has no equity interest in either company.
http://www.businesswire.com/news/home/20160516005711/en/Pfizer-Acquire-Anacor
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.