- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
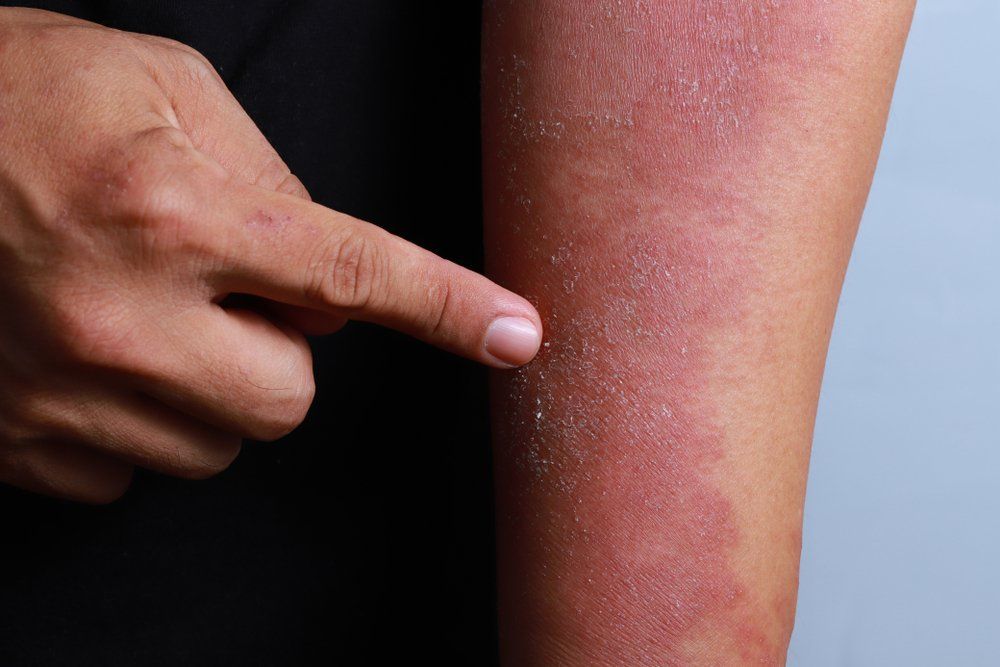
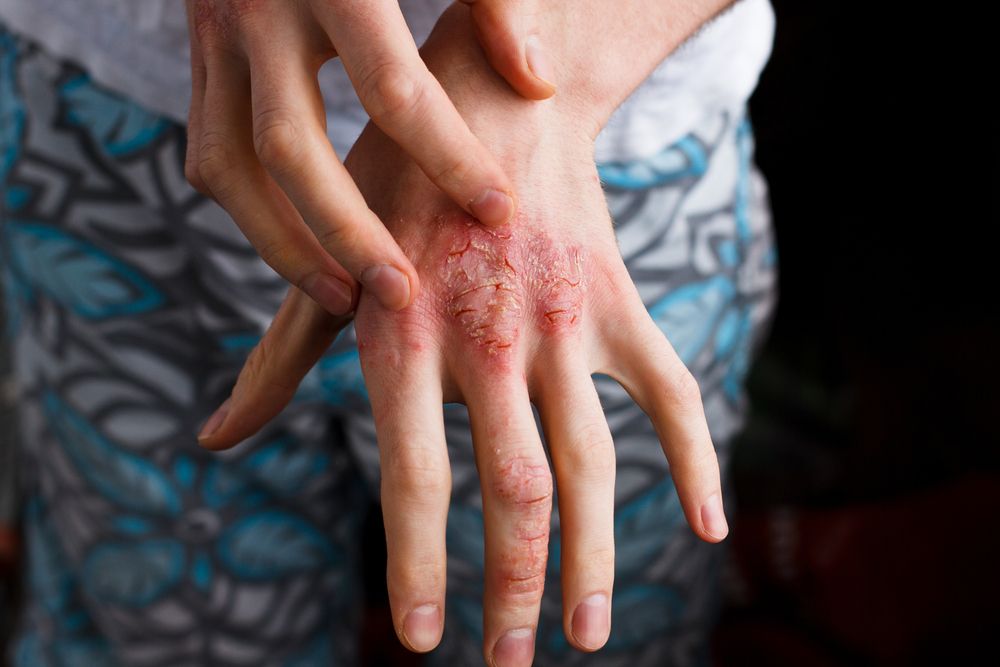
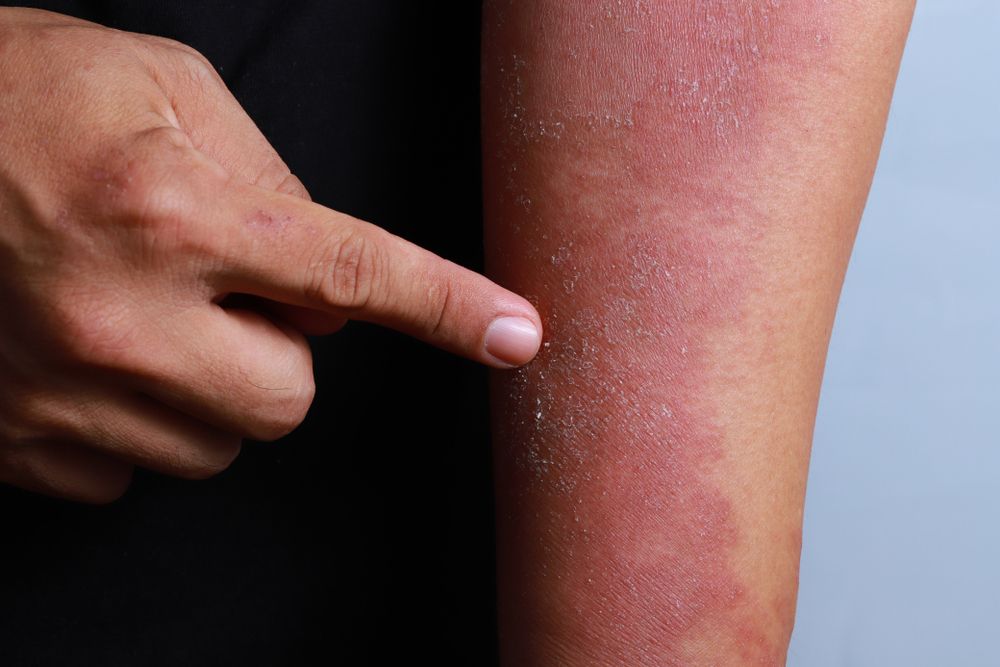
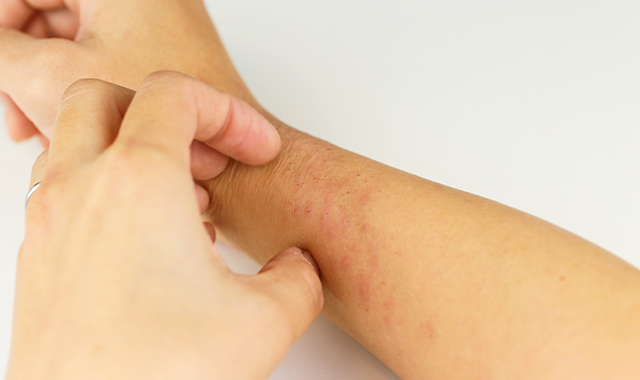
Methotrexate outperforms cyclosporine in atopic dermatitis study
Author(s):
The duration of methotrexate administration proves to be longer than that of cyclosporine in atopic dermatitis, according to a small study that compared the two first-line immunosuppressive treatments for atopic dermatitis.
“While drug survival analyses are increasingly used to study chronic treatments, including biotherapies in rheumatology and dermatology, post-drug survival is a new approach that addresses time free from any,immunosuppressive treatment, which is a main concern among patients," Journal of European Academy of Dermatology and Venereology, Man et al. Jan. 14, 2018
Methotrexate offers a better treatment profile than cyclosporin for long-term control of moderate-to-severe atopic dermatitis, according to a comparison study of two first-line immunosuppressive treatments.
Cyclosporine and methotrexate are first-line immunosuppressive treatments for adults and children with moderate-to-severe atopic dermatitis, used when the disease isn’t adequately controlled by topical medications and phototherapy. But long-term efficacy and safety concerns from cyclosporine and methotrexate presents major therapeutic challenges, wrote Catherine Droitcourt and French colleagues in the Jan. 14 issue of the Journal of the European Academy of Dermatology and Venereology
While researchers have analyzed drug survival to help determine how long patients with chronic atopic dermatitis can remain on immunosuppressive treatment with safe and effective outcomes, data is lacking on how long an atopic dermatitis patient can go without immunosuppressive treatment after it has been discontinued. The authors call this “post-drug survival,” or the time between drugs.
The researchers studied 56 patients at three French hospital dermatology departments, including 25 treated with cyclosporine and 31 with methotrexate. The patients, with moderate-to-severe atopic dermatitis were an average 34 years old, with equal numbers of men and women participating.
Researchers looked drug survival (defined as the length of time on the drug) and post-drug survival (the time between two drugs). Those treated with cyclosporine or methotrexate as first-tine immunosuppressive treatment for atopic dermatitis were part of the drug survival analysis, and patients who discontinued either drug made up the post-drug survival analysis.
Among the reasons for discontinuation - which did not differ significantly between cyclosporine and methotrexate use - were controlled disease, primary or secondary failure or side effects - none of which were considered serious and only occurred among three cyclosporine patients.
They found the median drug survival for cyclosporine was eight months, versus 23 for methotrexate. Six months after starting methotrexate, 93 percent of the patients studied were still on the drug, compared to 63 percent of patients on cyclosporine. At a year after initiating immunosuppression treatment, 71 percent of those on methotrexate were still on the drug, versus 38 percent in the cyclosporine group.
The only predicting factor for shorter drug survival was cyclosporine use.
The post-drug survival group included 18 patients who were on methotrexate and 20 on cyclosporine. Median post-drug survival was 12 months in the methotrexate group, versus two months for those who were on cyclosporine. None of the patients in the methotrexate group who had discontinued the drug because their disease was controlled needed a second-line treatment six months post-discontinuation, compared to 75 percent in the cyclosporine group.
The lone predictive factor for a shorter post-drug survival was cyclosporine use, the authors write.
Research has shown that comparative studies between common systemic treatments for atopic dermatitis are a research priority and big concern among clinicians. Yet, drug safety and efficacy in clinical studies, which include strict inclusion criteria, can be difficult to generalize to real-world, daily practice, they write.
And while drug survival analyses are increasingly used to study chronic treatments, including biotherapies in rheumatology and dermatology, post-drug survival is a new approach that addresses time free from any immunosuppressive treatment, which is a main concern among patients.
“We suggest including ‘post-drug survival’ for the purpose of better assessment in the use of immunosuppressive treatments, particularly in chronic skin diseases,” they write. “Taking into account both approaches, ‘drug survival’ and ‘post-drug survival,’ our findings suggest that [methotrexate] may present a better treatment profile for the long-term control of moderate-so-severe [atopic dermatitis], which is a well-identified challenge for clinical research and care.”
This study is limited in that it’s retrospective and has a small same size, the authors write.
The American Academy of Dermatology (AAD) recommendations for systemic immunomodulator use give cyclosporine and methotrexate a level B recommendation. Cyclosprine, with I and II evidence level, is recommended for atopic dermatitis patients who are refractory to conventional topical treatment, while methotrexate with folate supplementation is recommended for treatment of refractory atopic dermatitis and has level II evidence, according to AAD.
AAD recommendations note, however, there isn’t enough data to make strong recommends about optimal dosing, duration of therapy and precise monitoring protocols for systemic immunomodulating drugs.
REFERENCE
Sarah Law Ping Man, Guillaume Bouzillé, Nathalie Beneton, et al. "Drug survival and post-drug survival of first-line immunosuppressive treatments for atopic dermatitis: comparison between methotrexate and cyclosporine," Journal of the European Academy of Dermatology and Venereology. Feb. 14, 2018. DOI:10.1111/jdv.14880
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.