- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Minocycline as foam topical under study in acne
Author(s):
Study shows novel minocycline foam appears to be effective in moderate to severe acne vulgaris and is well-tolerated in phase three trials.
Study shows novel minocycline foam appears to be effective in moderate to severe acne vulgaris. (©AdobeStock_Frank_29052515)
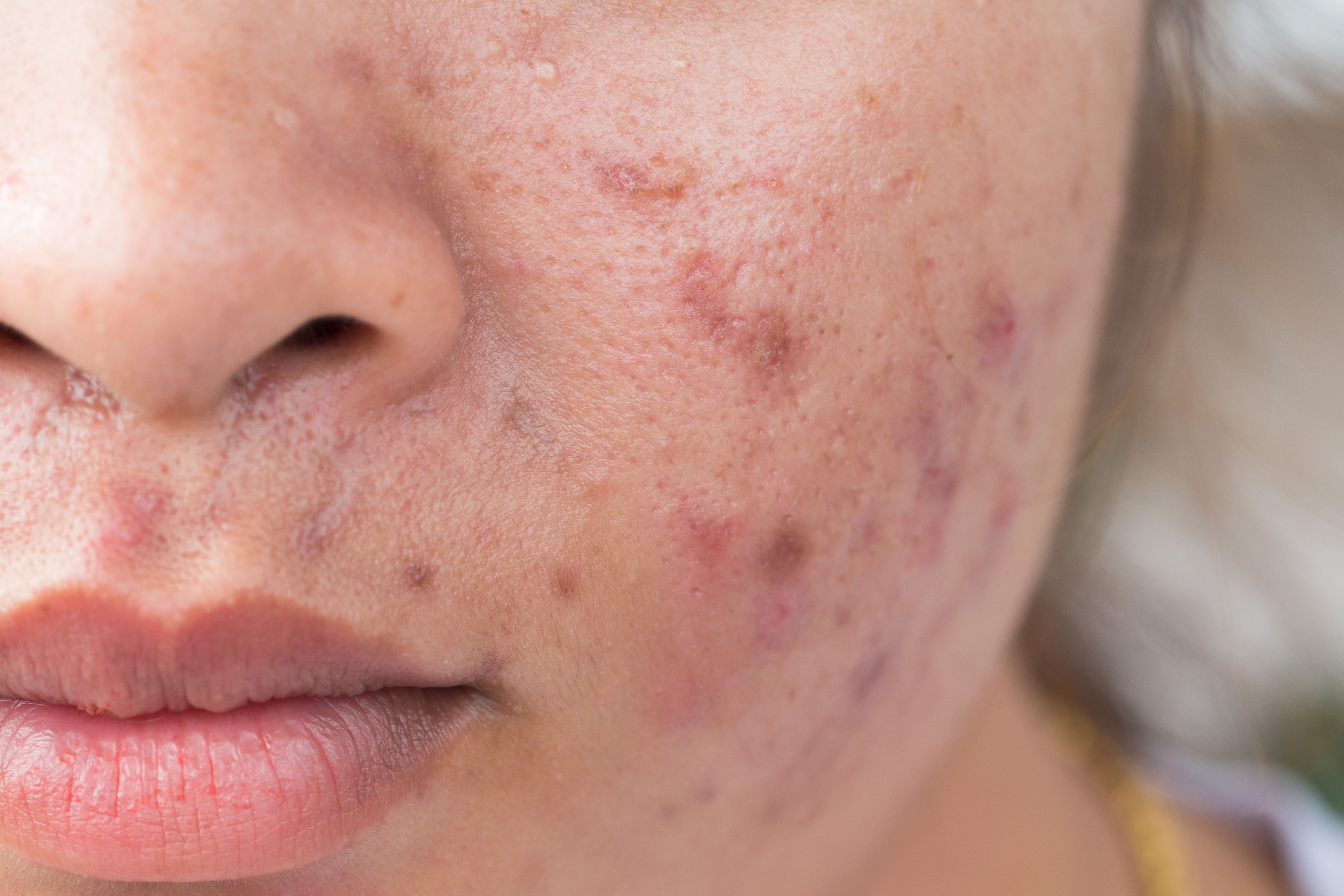
A novel topical minocycline foam has been found to significantly reduce both inflammatory and noninflammatory lesions and improve acne scores in patients with moderate-to-severe acne vulgaris in phase three clinical trials.
American Academy of Dermatology guidelines recommend minocycline and doxycycline as first-line therapies for moderate and severe acne. However, concerns about side-effects limit their use.
No topical minocycline is currently licensed but topical minocycline 4% foam has been tested in three phase three clinical trials and shown to produce significant improvements while minimising systemic side effects associated with oral administration.
A total of 961 patients were enrolled in the first two identical randomized double blind phase three trials (466 in trial 04 and 495 in trial 05) and randomized 2:1 to once-daily minocycline 4% foam or foam vehicle for 12 weeks.
The two co-primary endpoints were: the absolute change in the number of inflammatory lesions (papules and pustules); and the proportion of patients achieving treatment successes, defined as an Investigator’s Global Assessment (IGA) score of "clear" or "almost clear" and at least a two-grade improvement from baseline at week 12.
The results, published in the Journal of the American Academy of Dermatology showed that in both studies patients randomized to the active minocycline 4% foam experienced a significantly greater reduction in inflammatory lesions (P <0 .05). In study 04, the mean reduction in inflammatory lesions was -14.13 with the minocycline 4% foam compared with -11.19 with foam vehicle (P = 0.0083), and in study 05, lesion count fell by -13.46 with minocycline foam compared with -10.70 with foam vehicle (P = 0.0051). Significant reductions in non-inflammatory lesions were also seen with minocycline 4% foam in both studies (study 04: -16.45 vs -10.30 [P = 0.0042] and study 05: -13.20 vs -7.00 [P = 0.0320]).
However, only in study 05 did a significant number of patients achieve treatment success according to the Investigator’s Global Assessment (P < 0.05) - 14.66% using the minocycline 4% foam compared with 7.89% using the foam vehicle (P = 0.0424). In study 04 8.09% of patients treated with minocycline 4% foam and 4.77% treated with foam vehicle achieved treatment success (P = 0.2178)
Overall, minocycline 4% foam appeared to be safe and well tolerated across the two studies. A total of 113 adverse events were reported in 17.4% of the subjects in study 04, and 249 in 30.9% of the subjects in study 05 and most were mild or moderate. Skin-related adverse events were reported in less than 1% of patients.
One of the investigators Dr Linda Stein Gold from the Henry Ford Health System, Detroit, said poor adherence to acne treatment is a problem. “Systemic acne therapy may have lower adherence rates than topical therapy, potentially because of dissatisfaction arising from the lack of efficacy and the occurrence of side effects. The efficacy of the active drug and the potentially lower risk of systemic side effects with the topical minocycline foam may improve treatment adherence.”
Surveys of patients have indicated that in terms of topical treatments foams are generally preferred over creams, gels, and ointments. “The foam delivery system for topical drugs is easy to apply and does not leave a greasy or oily film on the skin after application,” Dr. Stein Gold said.
The investigators pointed out that, although IGA-assessed treatment success did not reach statistical significance in study 04, there was a clear numerical advantage for minocycline 4% foam, and a significant advantage was seen when data from both studies were pooled. The failure to reach clinical significance in study 04 could have been due to the small number of patients enrolled so a third phase 3 clinical trial with 1507 patients has taken place to address this.
Topline results from this third phase three trial show the mean reduction in inflammatory lesion count at week 12 relative to baseline was -16.93 for the minocycline 4% foam treatment group and -13.40 for the vehicle treatment group (p<0.0001). In addition, 30.80% of the active treatment group achieved IGA treatment success at week 12 compared with 19.63% for the vehicle treatment group (p<0.0001). There were no serious treatment related adverse events and minocycline 4% foam appeared to be safe and well-tolerated.
Foamix expects to make a new drug application to the FDA by the end of the year for minocycline 4% foam (candidate drug FMX101) in moderate to severe acne.
Iain A Stuart, senior vice president of research and development at Foamix, said that the data from this third confirmatory phase three study for minocycline 4% foam are “impressive” and that the strong body of clinical data generated “suggest that it may offer patients an efficacious treatment in a convenient and safe topical foam formulation.”
“If approved, it has the potential to address a significant unmet need in this difficult to treat condition,” he said.
REFERENCES
Gold LS, Dhawan S, Weiss J, Draelos ZD, Ellman H, Stuart IA. A novel topical minocycline foam for the treatment of moderate-to-severe acne vulgaris: Results of 2 randomized, double-blind, phase 3 studies Journal of the American Academy of Dermatology https://doi.org/10.1016/j.jaad.2018.08.020.)
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











