- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
More good news for corticosteroid combo treatment in atopic dermatitis
Author(s):
Study finds the combination of duplilumab and topical corticosteroids works in hard-to-treat atopic dermatitis cases in adult patients.
(©WarutChinsai/Shutterstock.com)
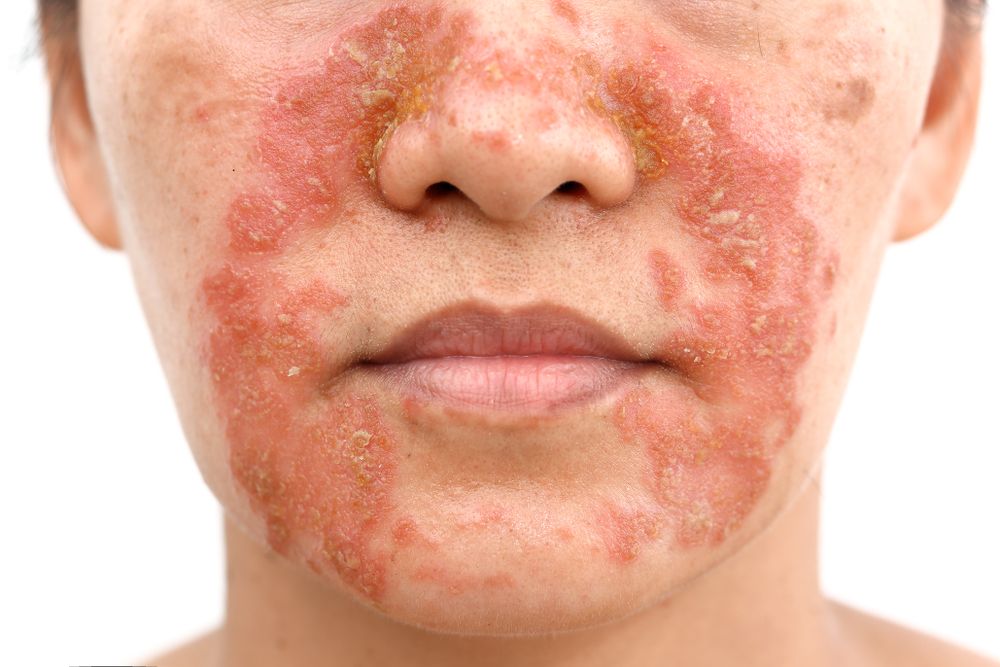
For adults with a moderate to severe case of atopic dermatitis that cannot be resolved with topical therapies, systemic immunosuppressants such as ciclosporin A are often the next step, but some patients cannot tolerate these agents and others have a poor response.
For those patients who can tolerate ciclosporin A, which suppresses Th1, TH2 and TH17/22, long-term use beyond one year is not recommended due to side effects, such as increased hypertension, nephrotoxicity and other side effects, such as headache, fatigue and tingling in fingers and toes.
So, researchers led by Marjolein de Bruin-Weller, M.D., Ph.D., of the University Medical Center Utrecht, the Netherlands, conducted a 16-week trial of dupilumab/topical corticosteroid treatment in adult atopic dermatitis patients who have a history of inadequate response or intolerance to ciclosporin A. They found that “dupilumab administered weekly or every two weeks with topical corticosteroids significantly improved signs and symptoms and quality of life, with no new safety signals.” The findings appear in the March 25 online issue the British Journal of Dermatology.
The study included 325 patients assigned to one of three groups: 300 mg dupilumab once weekly (110 patients), dupilumab once every two weeks (107 patients) and placebo (dummy drug) once weekly (108 patients).
At baseline, the Eczema Area and Severity Index (EASI) scores were 31 (scale: 0-72) for all patients. By week 16, 59.1 percent of the once-weekly dupilumab group and 62.6 percent of the once every other week attained at least a 75 percent improvement in EASI, compared to only 29.6 percent of patients on placebo.
“These results are consistent with those of previous studies of dupilumab in patients with moderate-to-severe atopic dermatitis inadequately controlled with topical medications,” the authors wrote.
The combination treatment of dupilumab and topical corticosteroid treatment improved itch, mood and quality of life as well. The proportion of patients who achieved below eight (one a scale of 0-21) on the anxiety and depression subscores of the Hospital and Depression Scale (HADS) was significantly greater in the group that was given dupilumab every other week versus once a week. Both dupilumab regimens demonstrated significant improvement in mean change in HADS score from baseline.
“These data support the use of dupilumab in this difficult-to-treat population,” the authors wrote.
ADVERSE EVENTS
The presence of conjunctivitis was noteworthy in this study with 11-28 percent of patients in the treatment groups experiencing symptoms, although most were mild.
In other studies of other diseases treated with dupilumab, including asthma and chronic rhinosinusitis with nasal polyposis, the treatment was not linked to increased rates of conjunctivitis. The increased rates of conjunctivitis in atopic dermatitis studies may be due to a unique interaction between the mechanisms of action of atopic dermatitis and dupilumab, the authors wrote.
Herpes viral infections, was reported in 7 percent, 5 percent and 6 percent of patients in each of the three groups, respectively; however, the most serious forms of these infections were limited to the placebo group.
Safety of the drug beyond one year is being evaluated in an open-label extension study.
In terms of limitations, the study was not designed to compare the two dupilumab treatment protocols. And, the study was not conceived to compare cyclosporine A subgroups of treated and naïve patients.
DISCLOSURES
The research was sponsored by Sanofi and Regeneron Pharmaceuticals, Inc.
REFERENCE
de Bruin-Weller M, Thaci D, Smith CH, et al. “Dupilumab With Concomitant Topical Corticosteroid Treatment in Adults With Atopic Dermatitis With an Inadequate Response or Intolerance to Ciclosporin A or When This Treatment is Medically Inadvisable: a Placebo-controlled, Randomized Phase III Clinical Trial (LIBERTY AD CAFÃ),” British Journal of Dermatology, 178, 1083-1101, May 2018. DOI:org/10.1111/bjd.16623





