- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Multi-Omics Psoriasis Treatment Approach
Author(s):
Study authors hypothesize that a monoclonal blockade of pathogenic T cells may induce expansion of regulatory immune cells subsets or expression of cytokines involved in skin homeostasis.
Milan Lipowski/Adobe Stock
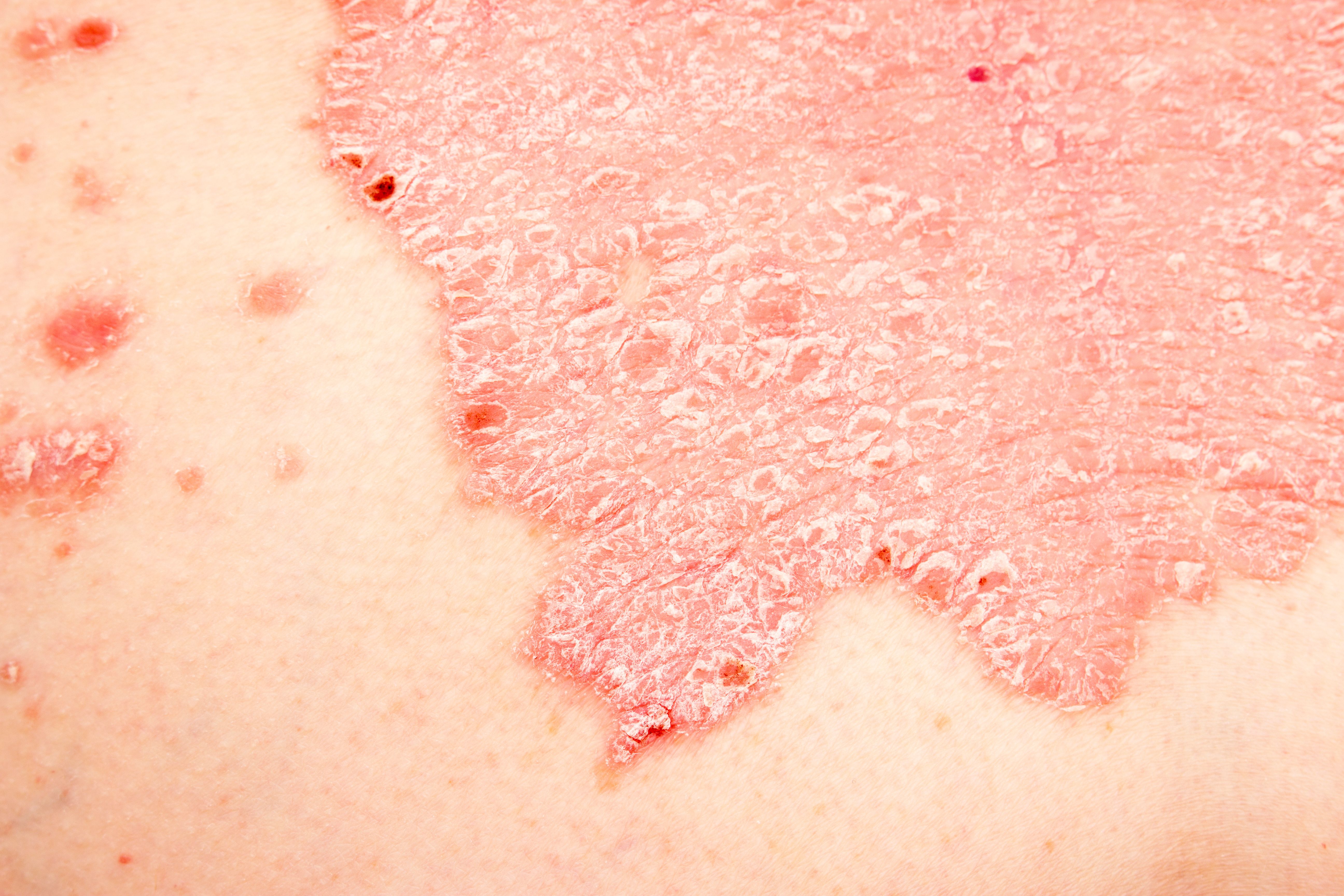
Shown to be highly selective for the IL-17A proinflammatory cytokine, secukinumab (Cosentyx) is a human monoclonal antibody that has demonstrated a high efficacy in quelling moderate to severe psoriasis symptoms. A recent study1 looked at the therapeutic benefits in psoriasis treatment when using a multi-omics approach combining immune cell-enriched single-cell transcriptomics (scRNA-seq), microarray analysis, and immunohistochemistry to better understand the translational role of anti–IL-17A in suppressing the autoimmune functions of type 17 T (T17) cells.
Occurring in up to approximately 8 million adults in the United States alone, psoriasis remains challenging to treat and can often be the cause of decreased self-esteem, depression, social stigma, and shame, significantly impacting the quality of life of patients. The disease is characterized by immune-mediated inflammation and abnormal keratinocyte differentiation, resulting in the typical clinical presentation of silver-white scaling, erythematous macules, papules, and increasingly thickening plaques.
In addition to the cutaneous manifestations, psoriasis is associated with a number of comorbidities including metabolic abnormalities, psoriatic arthritis, vascular inflammation, diabetes, and cardiovascular disease, all of which can further lead to significant reductions in the patient’s health, quality of life, and overall life span. This underscores the necessity, and urgency, of finding more effective treatment options that can help circumvent the short- and long-term consequences of psoriatic disease to improve quality of life as well as decrease the risk of comorbid disease in patients with psoriasis.
Advancements in Treatment Options
Data from immunologic and genetic studies have identified IL-17 and IL-23 as key drivers of psoriasis pathogenesis, and the targeting of these cytokines has significantly improved the treatment and management of patients with psoriasis.
Some biologic medications that are currently approved for the treatment of moderate to severe psoriasis include TNF-alpha inhibitors (adalimumab, etanercept, infliximab), IL-17 pathway inhibitors (ixekizumab, brodalumab, secukinumab), IL-12/IL-23 inhibitors (ustekinumab), and IL-23 inhibitors (guselkumab, tildrakizumab), with each agent having its unique efficacy and safety profile. It is thought that TH17 lymphocytes, differentiating under the influence of dendritic cell-derived IL-23 and mediating their effects via IL-17A, are the central effector cells in psoriasis, a notion supported by the high efficacy of those therapeutic agents that target this pathogenic axis.
Studying a Multi-Omics Approach
In the study, 23 adult patients with psoriasis received secukinumab 300-mg injections administered once weekly for at least 12 weeks. They received treatment at baseline; at weeks 1, 2, 3, and 4; and then every 4 weeks after that. Researchers analyzed and compared 6-mm punch biopsy tissues from psoriatic skin before, and 12 weeks after, systemic IL-17A blockade using the multigenomics approach integrating immune cell–enriched scRNA-seq (n = 18), microarray (n = 61), and immunohistochemistry (n = 61) with repository normal control skin immune cell–enriched scRNA-seq (n = 10) and microarray (n = 8) data.
It was found from the data that systemic IL-17A blockade depleted 100% of IL-17A–positive (+) T cells and 95% of IL-17F + T cells in psoriasis skin and caused significant downregulations in IL-23A gene expression in dendritic cell subsets, leading to a long-term reduction in psoriasis symptoms, even after the medication had been halted.
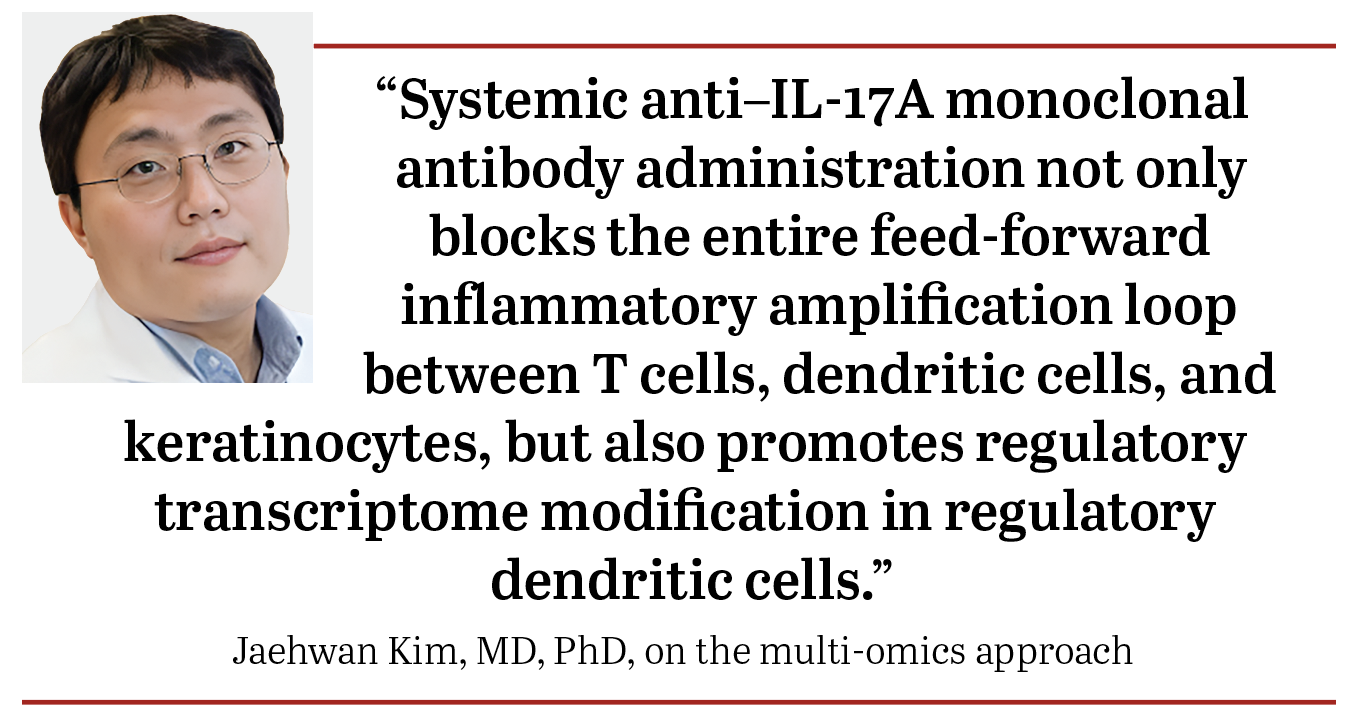
“Our multi-omics approach provides novel translational evidence in human psoriasis skin that systemic anti–IL-17A monoclonal antibody administration not only blocks the entire feed-forward inflammatory amplification loop between T cells, dendritic cells, and keratinocytes, but also promotes regulatory transcriptome modification in regulatory dendritic cells,” wrote lead study author Jaehwan Kim, MD, PhD, and colleagues including James Krueger, MD, Laboratory for Investigative Dermatology, The Rockefeller University, New York, New York. Kim and Krueger have been part of extensive research for years on the immunopathogenesis of psoriasis, innovations for psoriasis treatment options, and gaining insight from patient experiences.
The study data also showed that the expression of IL-17–driven inflammatory mediators (IL36G, S100A8, DEFB4A, and DEFB4B) in suprabasal keratinocytes was correlated with psoriasis severity and was downregulated by IL-17A blockade. In addition, the authors noted that IL-17A blockade induced a higher gene expression of CD1C and CD14, which are markers of CD1c + and CD14+ dendritic cell subset that suppresses antigen-specific T-cell responses in posttreatment regulatory semimature dendritic cells compared with pretreatment regulatory semimature dendritic cells.
Previous studies have shown that when patients with moderate to severe psoriasis received systemic anti–IL-17A monoclonal antibody (secukinumab) treatment for 52 weeks and then stopped treatment, 16% maintained a response after treatment withdrawal over the next 52 weeks without need for re-treatment.2,3
“Our study [data] may explain how those patients restored immune tolerance to psoriasis autoantigens that may have prevented recurrence of psoriasis off the treatment. In addition to the co-modulation of IL-17A and IL-17F by direct and indirect effects on T cells including IL-23 reduction in dendritic cells, systemic IL-17A blockade may have effectively modified regulatory transcriptome of regulatory dendritic cells in those patients,” the study authors wrote.
Opportunities for Further Research
Looking further ahead, the authors hypothesize that a monoclonal blockade of pathogenic T cells, such as an IL-17A blockade or an IL-23p19 blockade, may induce expansion of regulatory immune cells subsets or expression of cytokines involved in skin homeostasis. Kim and colleagues are currently investigating this hypothesis in an ongoing clinical trial to better understand the regulatory immune cell promotion by blocking the IL-23/IL-17 cell axis and hopefully develop more personalized medicine.
References
- Kim J, Lee J, Li X, et al. Multi-omics segregate different transcriptomic impacts of anti-IL-17A blockade on type 17 T cells and regulatory immune cells in psoriasis skin. Front Immunol. 2023;14:1250504. doi:10.3389/fimmu.2023.1250504
- Langley RG, Sofen H, Dei-Cas I, et al. Secukinumab long-term efficacy and safety in psoriasis through to year 5 of treatment: results of a randomized extension of the phase III ERASURE and FIXTURE trials. Br J Dermatol. 2023;188(2):198-207. doi:10.1093/bjd/ljac040
- Blauvelt A, Reich K, Warren RB, et al. Secukinumab re-initiation achieves regain of high response levels in patients who interrupt treatment for moderate to severe plaque psoriasis. Br J Dermatol. 2017;177(3):879-881.doi:10.1111/bjd.15656
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







