- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
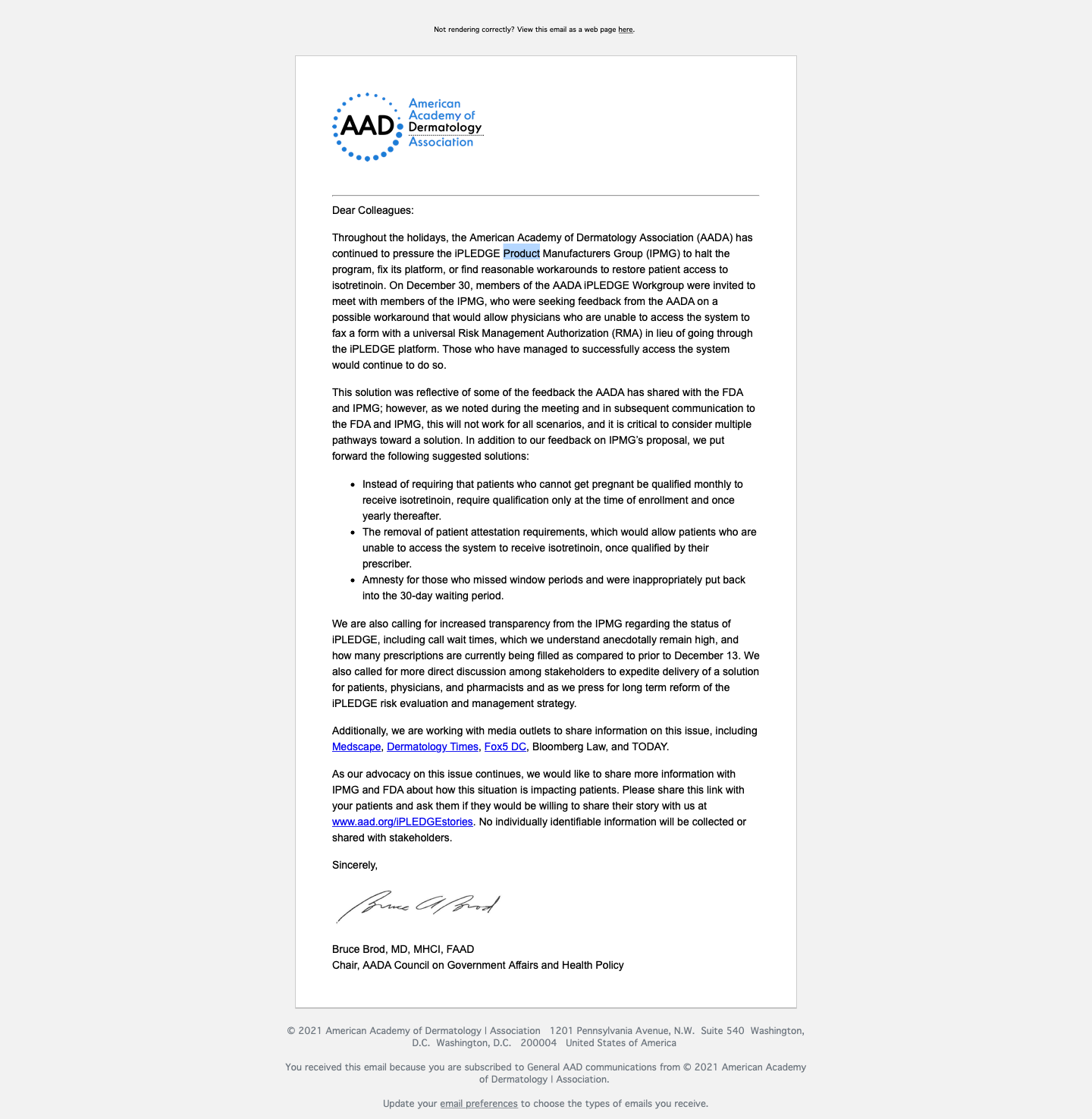
Nearly Two-Thirds Still Locked Out of iPLEDGE
Author(s):
A survey conducted by Dermatology Times® found that many still cannot access the iPLEDGE system. Now some have created guides to get around the issue.
The iPLEDGE Risk Evaluation and Mitigation Strategy (REMS) went through FDA-approved modifications, including system changes for health care professionals and patients which took effect December 13, 2021. The website changes were announced by the FDA on October 8, 2021, stating that it had approved a modification to the iPLEDGE REMS which would take effect on that December 13, 2021, date. This has led to many not being able to dispense or receive their isotretinoin medication.
In the weeks following the change many health care practitioners and patients have had issues accessing the site. A recent poll done on Dermatology Times® found that two-thirds of survey takers were locked out of the system, while an additional poll found that over 95% of responders agreed the iPLEDGE system should be discontinued.
A statement from the American Academy of Dermatology Association (AADA), featured below, explained how the organization has been working with the (IPMG) to “halt the program, fix its platform, or find reasonable workarounds to restore patient access to isotretinoin.”
Statement provided to Dermatology Times® from the AADA
Statement provided to Dermatology Times® from the AADA
The AADA released a letter yesterday stating in their tweet that, “The AADA continues to pressure the #iPLEDGE Product Manufacturers Group (IPMG) to halt the program, fix its platform, or find reasonable workarounds to restore patient access to isotretinoin. Learn more, plus how patients can submit their stories.”
AADA Statement from Twitter
There have been attempts by some to find work arounds into the system. One such workaround, created by Meghan Keating, an isotretinoin patient, and retweeted by John Barbieri, MD, MBA, a member of the AADA iPLEDGE Workgroup, lists instructions to help patients unable to log into the site. The instructions feature 5 optional ways for patients to try to log in to the system.
There is also a Change.org petition called, "Cancel iPLEDGE REMS Program so that accutane patients can get their prescription meds," that has been circulating as many have been locked out of getting medication for weeks.
As of now, no new information from the FDA has been announced on whether the program will be halted or discontinued as patients still attempt to get their isotretinoin medication.
Stay tuned for continued coverage from the Dermatology Times® as this situation evolves.
Reference:
Ipledge risk evaluation and mitigation strategy (REMS). FDA. Published online December 14, 2021. Accessed January 4, 2021. https://www.fda.gov/drugs/postmarket-drug-safety-information-patients-and-providers/ipledge-risk-evaluation-and-mitigation-strategy-rems
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











