- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
NEJM Publishes Data on the Application of B-VEC to Treat Ocular Complications in Patients with DEB
Author(s):
Krystal Biotech’s B-VEC was administered as an eyedrop for a patient with dystrophic epidermolysis bullosa with cicatrizing conjunctivitis.
Alessandro Grandini/AdobeStock
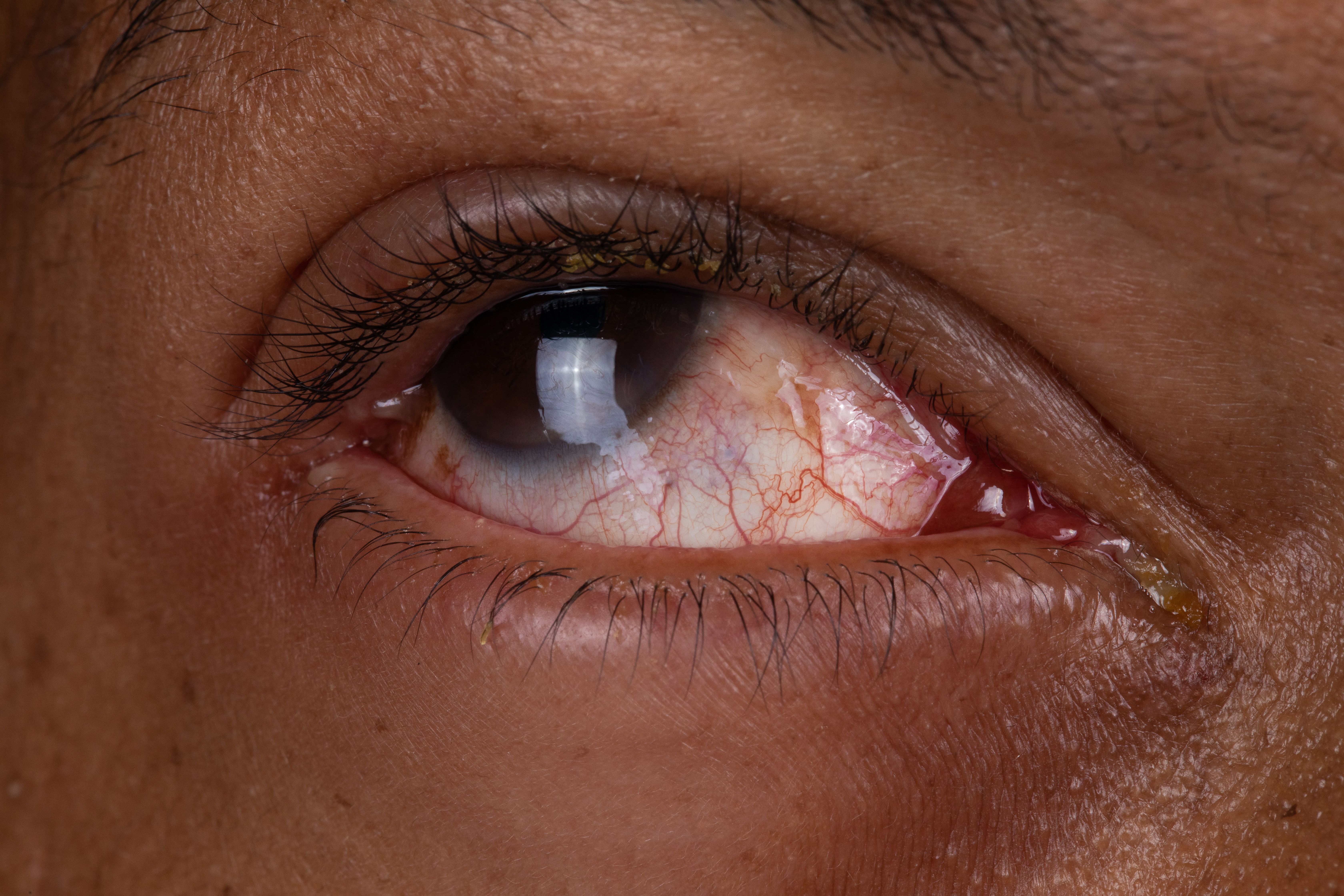
Krystal Biotech recently announced the publication of new data in the New England Journal of Medicine on the use of beremagene geperpavec (B-VEC), administered as an eyedrop to treat a patient with dystrophic epidermolysis bullosa (DEB) with cicatrizing conjunctivitis.1 In the study, “Ocular Gene Therapy in a Patient with Dystrophic Epidermolysis Bullosa,” the authors reported the case of a patient with severe cicatrizing conjunctivitis in both eyes.2
“DEB is a devastating disease and patients with ocular complications have no corrective treatment options leaving them at risk of severe vision loss,” said Alfonso L. Sabater, MD, PhD, associate professor of clinical ophthalmology at the Bascom Palmer Eye Institute at the University of Miami Miller School of Medicine, and study investigator, in the news release. “We are encouraged by the improvements observed in the patient following B-VEC administration as an eyedrop directly to the affected eye and believe this data is supportive of further investigation in DEB patients with ocular complications. If approved, this approach could drastically benefit these patients.”
According to Krystal Biotech, over 25% of patients with DEB develop ocular complications such as corneal erosions, abrasions, blistering, and scarring that can cause impaired vision. The management of DEB varies from supportive care and wound management to surgical interventions to remove scar tissue. However, no corrective therapy is currently available.1
The patient from the study presented with severe cicatrizing conjunctivitis secondary to DEB. Surgical symblepharon lysis of the patient’s right eye with pannus removal was performed and regular administration of B-VEC as an eyedrop directly to the eye (5×109 PFU/mL) was added to the routine post-surgical care, which included 3 times per week for the first 2 weeks and then once weekly. The application frequency of B-VEC was then decreased to once a month after the corneal epithelium was healed. B-VEC was well tolerated and associated with full corneal healing by 3 months and significant visual acuity improvement from hand motion to 20/25 at 8 months.
“We are excited by this initial data suggesting additional applications of our proprietary HSV-1-based gene therapy platform to treat ocular diseases, and we are working with the FDA to get B-VEC approved for the treatment of DEB patients with lesions in the eye,” said Suma Krishnan, president of research and development at Krystal Biotech.
According to the data, ocular complications are common in patients with DEB, with over half of the patients diagnosed with recessive DEB potentially affected. Currently, there are no FDA-approved treatment options for ocular manifestations of DEB.
B-VEC was FDA-approved for the treatment of DEB in May 2023 based on data from 2 placebo-controlled clinical trials, GEM 3 (NCT04491604) and GEM1/2 (NCT03536143). B-VEC is a non-invasive, topical, redosable gene therapy created to administer two copies of the COL7A1 gene when directly applied to DEB wounds. B-VEC treats DEB at the molecular level by giving patients’ cells a template to produce normal COL7 proteins. The FDA granted B-VEC orphan drug designation, fast track designation, pediatric designation, and regenerative medicine advanced therapy for the treatment of DEB.3
References
- Krystal Biotech announces publication in the New England Journal of Medicine on the application of B-VEC to treat ocular complications in patient with dystrophic epidermolysis bullosa. News release. Krystal Biotech. February 8, 2024. Accessed February 8, 2024. https://ir.krystalbio.com/news-releases/news-release-details/krystal-biotech-announces-publication-new-england-journal
- Tovar Vetencourt A, Sayed-Ahmed I, Gomez J, et al. Ocular Gene Therapy in a Patient with Dystrophic Epidermolysis Bullosa. N Engl J Med. 2024;390(6):530-535. doi:10.1056/NEJMoa2301244
- FDA approves first topical gene therapy for treatment of wounds in patients with dystrophic epidermolysis bullosa. U.S. Food and Drug Administration. May 19, 2023. Accessed February 8, 2024. https://www.fda.gov/news-events/press-announcements/fda-approves-first-topical-gene-therapy-treatment-wounds-patients-dystrophic-epidermolysis-bullosa.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







