- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
New Investigational Topical Peptide Offers Promising Eczema Treatment
Author(s):
Cytokine-expressing genes were silenced in a recent Vanderbilt University Medical Center study.
Researchers at Vanderbilt University Medical Center (VUMC) say their topical peptide drug controls the 15 genes responsible for the painful inflammation that comes with atopic dermatitis (AD)1. This topical treatment by a Nuclear Transport Checkpoint Inhibitor (NTCI) targets two nuclear transport shuttles that suppress the expression of keratinocyte-derived cytokine, Thymic Stromal Lymphopoietin (TSLP)—the key gene in AD development.
The drug’s study was conducted on mice in strict compliance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health. An AD phenotype was induced in 8-week-old C57 black 6 female mice. The 4 experimental groups (Mock Control, n=3; MC903 Untreated, n=5; MC903+Saline, n=5; and MC903+cSN50.1, n=5) were randomly selected. The AD-like phenotype was applied to the right ear of each mouse for 21 days. NTCI treatment was introduced on day 20 twice each day for 9 days. The saline control represented a vehicle for all the NTCI peptide used in this study. Each experiment was performed twice to ensure significance and experimental reproducibility as the topical peptide continues testing. All mice were euthanized on day 29 by over inhalation of isoflurane followed by cervical dislocation. The ear samples from the experimental mice were collected postmortem to isolate total RNA and evaluate genetic differences among experimental groups.
Representative pictures of mice from the mock control group were taken at the end of experiment and from the cSN50.1-treated group immediately before (MC903 AD Induction) and after NTCI treatment (MC903 + cSN50.1).
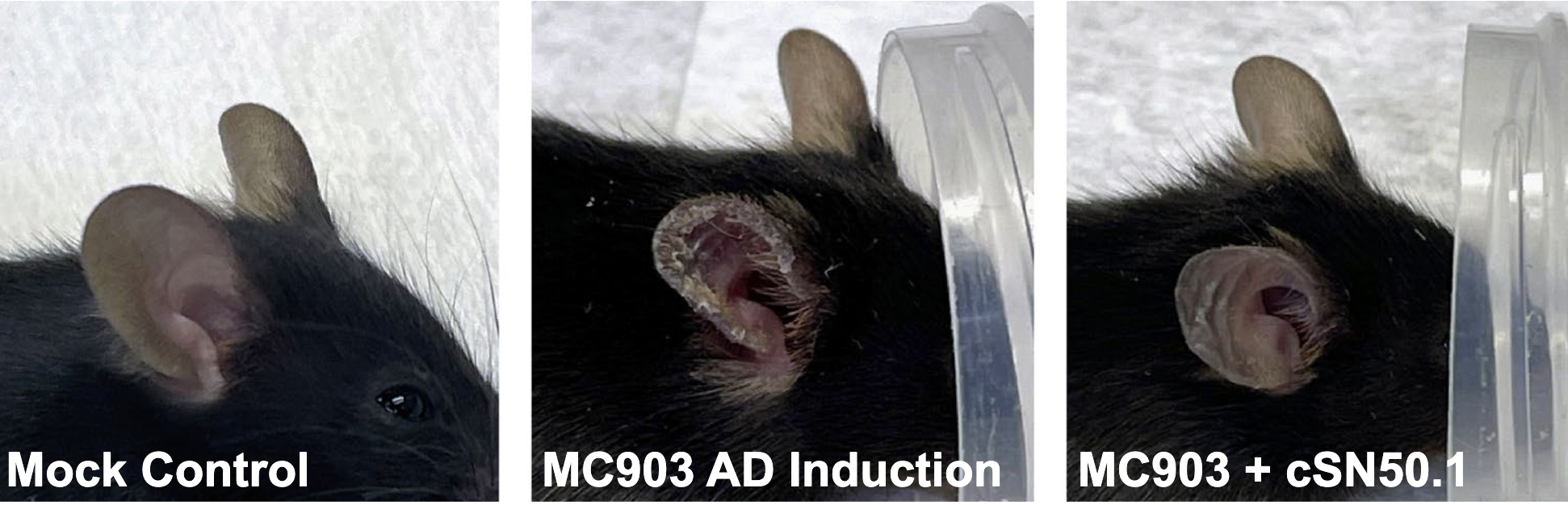
Study researchers report the NTCI, cell-penetrating cSN50.1 peptide is a novel agent for the treatment of eczema. The experimental drug’s vitamin D3 analog was also found to induce the signs of AD during its clinical trial in patients with psoriasis, which led to this testing. Study results show NTCI suppresses the gene encoding TSLP, and can drastically help reduce itching, inflammation, and open skin lesions without apparent toxicity.
The investigative topical peptide was coinvented and patented by VUMC researchers, one of which cofounded Amytrx Therapeutics, Inc. Jacek Hawiger, MD, PhD says this study has led to the topical drug now being tested in a multicenter clinical trial among patients with eczema.
Reference
1. Liu Y, Zienkiewicz J, Qiao H, et al. Genomic control of inflammation in experimental atopic dermatitis. Sci Rep. 2022;12(1):18891. Published 2022 Nov 7. doi:10.1038/s41598-022-23042-x
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











