- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Nitric oxide-releasing platform shows woundcare potential
Researchers developed a nitric oxide-releasing nanotechnology platform and have investigated its efficacy for treating full-thickness wounds infected with MRSA.

Key Points
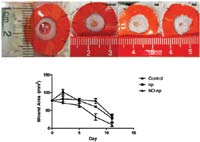
In the study, animals with excisional wound MRSA infections were divided into three groups and were treated topically with the NO nanoparticles, were treated topically with the nanoparticles alone, or were left untreated.
Follow-up with clinical, photographic and microbiological assessment of the infected wounds through day seven postinfection showed the NO-nanoparticle treatment had bactericidal activity and accelerated wound closure. Histological assessment confirmed that it eliminated bacterial burden as well as decreased suppurative inflammation and minimized collagen degradation.
"All of the components used in our platform are found on the GRAS (Generally Recognized as Safe) list, and so far it has also been shown to be completely nontoxic in comprehensive safety testing conducted in both in vitro and in animal models. Our research and development efforts are continuing with the hope of bringing this promising technology to the bedside," he adds.
An ingenious method
Richard B. Weller, M.D., senior lecturer, department of dermatology, University of Edinburgh, Scotland, is a leading researcher on the effects of NO on the skin. In a commentary accompanying the published study, Dr. Weller notes that the Albert Einstein College of Medicine team had developed "an ingenious method of storing NO."
"This is an interesting approach to the problem of storing nitric oxide in a form which allows release at the desired site of action," Dr. Weller says. "The group from Albert Einstein College has shown promising data for the use of their technology in the treatment of infected wounds."
The nanoparticle platform is a hybrid composite, combining properties of silane-based hydrogels and sugar-derived glasses. An initial evaluation using real-time amperometric analysis to determine its performance in releasing NO showed that it provided controlled and sustained delivery for greater then 24 hours.
Microbial and wound-healing studies were then performed under the guidance and leadership of Joshua Nosanchuk, M.D., associate professor in the departments of medicine and microbiology and immunology at Albert Einstein College of Medicine. In evaluating the in vitro antimicrobial activity of the platform against 20 clinical strains of S. aureus, including 11 MRSA strains, the results indicated the bacteria were 100 percent susceptible, and monitoring with transmission electron microscopy documented a series of cellular changes culminating in complete bacterial cell lysis within 24 hours.
In the animal study, 5 mm punch biopsy wounds were created and inoculated with 107 colony-forming units of MRSA. Clinical evaluation at both days three and seven showed that the wounds in the NO-nanoparticle treatment group exhibited less erythema, induration and crusting and were also significantly smaller than in both control groups.
Histological assessment of tissue removed at seven days demonstrated absence of bacteria, less inflammatory infiltrate and cellular necrosis and evidence of accelerated wound healing with the presence of increased fibrin deposition.
"It is well-known that after the inflammatory stage of wound healing is completed, NO aids in the recruitment and mobilization of keratinocytes and fibroblasts, stimulates both the production of collagen and angiogenesis, and directs wound closure. While our study methods have not proven these biological effects, our evaluations of potential mechanisms suggest that the NO treatment protected against collagen destruction by eradicating the bacteria and reducing infiltration of inflammatory cells," Dr. Friedman says.
Moving ahead
Current and future research plans for developing the NO-releasing nanoparticles as a treatment for cutaneous infections are extensive and include investigations of its effects on wound healing beyond seven days, as well as in the setting of comorbidities such as diabetes. Its efficacy in treating infections of varying tissue depths or caused by other resistant pathogens is planned for study as well.
"One of the benefits of nanotechnology is the opportunity for enhanced skin penetration, which suggests our platform may be useful for treating deeper infections, such as abscesses," Dr. Friedman notes.
Recognizing that medical comorbidity is a common feature in patients with refractory wounds, studies are also being planned using diabetic and immunocompromised animal models. Investigators are currently evaluating the efficacy of the NO-releasing nanoparticles against pathogenic fungi, malaria, Mycobacterium, leishmaniasis and other multi-drug- resistant bacteria.
"NO has broad-spectrum antimicrobial activity because it is directly cytotoxic. While there is some evidence that resistance can develop - for example, certain species of Staphylococcus have been reported to generate an enzyme that can scavenge NO produced by the body - we believe this issue can be easily overcome with an extrinsically delivered source of NO, where we can manipulate the amount and duration of NO exposure," Dr. Friedman says.
"Not only can our nanoparticle platform be modified to regulate the amount of NO release, but as another attractive feature, surface treatments can be applied to achieve tissue specific targeted delivery," he adds.
Dr. Friedman emphasizes that the project represents a multidisciplinary collaboration, including members from the division of infectious disease, department of microbiology and immunology and department of physiology and biophysics.
Dr. Friedman is co-senior author of the published paper together with Dr. Nosanchuk and Joel Friedman, M.D., Ph.D., department of physiology and biophysics, co-inventor of the nanotechnology.
Dr. Friedman also wishes to recognize the contributions of Luis Martinez, Ph.D., and George Han, M.S., who are co-first authors and members of the collaborative program.
Disclosures: Dr. Friedman is on the scientific advisory board of Makefield Therapeutics, a privately held biotechnology company that has licensed this nanotechnology from Albert Einstein College of Medicine.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











