- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Novan Submits NDA for Berdazimer Gel for the Treatment of Molluscum Contagiosum
Author(s):
Potential FDA approval is expected in the first quarter of 2024.
Novan, Inc. has submitted a New Drug Application (NDA) to the US Food and Drug Administration (FDA) for the approval of berdazimer gel, 10.3% for the topical treatment of molluscum contagiosum. Novan is expecting an early 2024 FDA approval. The NDA submission of berdazimer gel is also Novan’s first NDA submission to the FDA.1
The NDA submission is based on positive data from phase 3 studies of berdazimer gel, 10.3%, B-SIMPLE4 (NCT04535531). Berdazimer gel demonstrated statistically significant improvement in the primary endpoint of complete clearance of all treatable molluscum contagiosum at week 12. Berdazimer gel was reported to be well tolerated with mild application site pain and mild to moderate erythema reported as the most common adverse event.
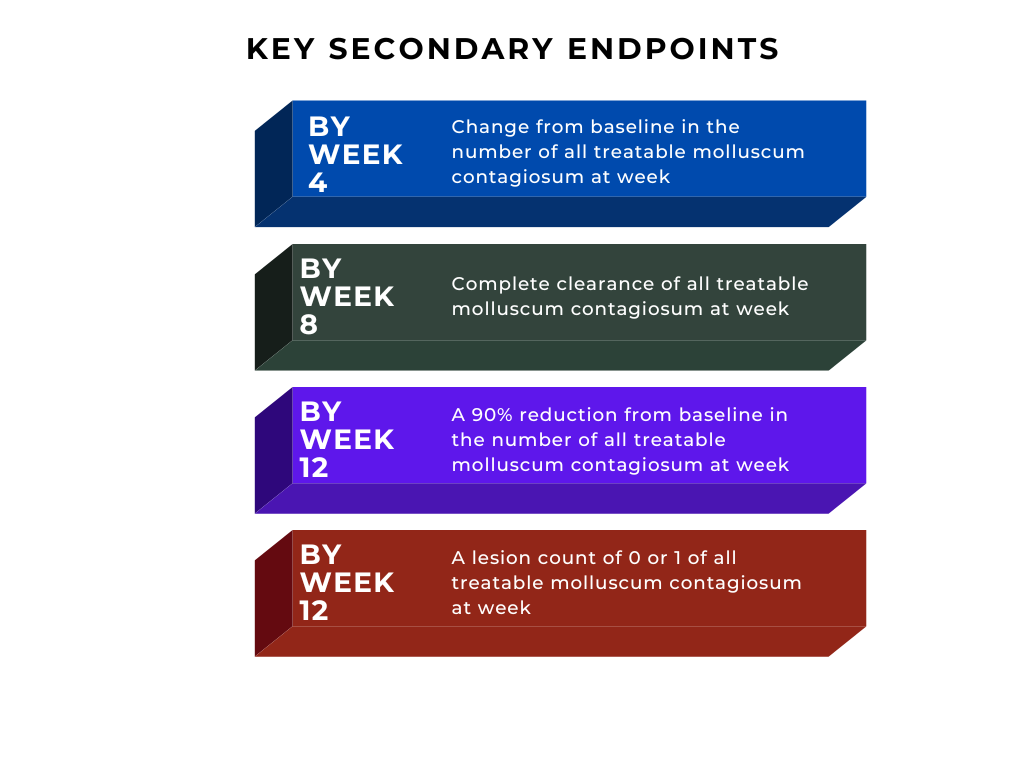
See Figure 1 for key secondary endpoints:2
Figure 1
According to Adelaide Hebert, MD, chief of pediatric dermatology at McGovern School of Medicine and Children’s Memorial Hermann Hospital, many pediatric patients with molluscum contagiosum go untreated due to a lack of FDA-approved treatments. A topical gel like berdazimer is easier for patients or caregivers to apply, while still effectively clearing molluscum lesions without an in-office visit.
The active ingredient in berdazimer gel, 10.3% is berdazimer sodium, a new chemical entity. Berdazimer gel for the treatment of molluscum contagiosum is a potential first-in-class topical nitric oxide-releasing agent.
Molluscum contagiosum is a common, contagious viral skin infection caused by the molluscipoxvirus, affecting approximately 6 million people in the US, with the greatest incidence in pediatric patients aged one to 14 years. Infected children typically present with 10 to 30 lesions, and in severe cases, they can have around 100 lesions, mostly appearing on the face, trunk, limbs, and axillary areas. Over 70% of molluscum patients go untreated.
References
- Novan submits new drug application to the US FDA for berdazimer gel, 10.3% (SB206) for the treatment of molluscum contagiosum. Novan Inc. Published January 6, 2023. Accessed January 6, 2023. https://novan.com/novan-submits-new-drug-application-to-the-u-s-fda-for-berdazimer-gel-10-3-sb206-for-the-treatment-of-molluscum-contagiosum/
- A phase 3 molluscum contagiosum efficacy and safety study (B-SIMPLE4). ClinicalTrials.gov identifier: NCT04535531. Updated August 13, 2021. Accessed January 6, 2023. https://clinicaltrials.gov/ct2/show/NCT04535531?cond=molluscum+contagiosum&draw=2&rank=2
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.