- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Novel cephalosporin fights MRSA
Washington — Promising results were seen in a phase 2 trial of the first drug in a new class of cephalosporins active against multi-drug resistant Staphlococcus aureus, according to Markus Heep, M.D., medical microbiologist and project physician at Basilea Pharmaceutica, Ltd. in Basel, Switzerland.
Washington - Promising results were seen in a phase 2 trial of the first drug in a new class of cephalosporins active against multi-drug resistant Staphlococcus aureus, according to Markus Heep, M.D., medical microbiologist and project physician at Basilea Pharmaceutica, Ltd. in Basel, Switzerland.

Novel cephalosporinCeftobiprole medocaril is a novel cephalosporin with potent activity in vitro against both methicillin-resistant S. aureus (MRSA) and methicillin-sensitive S. aureus MSSA).
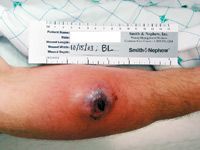
"Bacteria that cause cSSSIs,especially MRSA, have been shown to be able to counter all currentlyavailable drugs including newer arrivals like linezolid, daptomycin and tigecycline. A drug that restoresactivity of the well-established cephalosporin class would be avaluable asset in this setting. Ceftobiprole has this potential: bactericidal anti-MRSA activity combinedwith the spectrum of third or fourthgeneration cephalosporins."
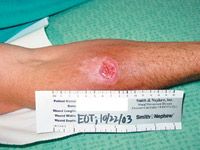
The study was designed to evaluate efficacy after treatment duration of at least seven days, with treatment prolongation as long as 21 days permitted. At seven to 10 days after the end of treatment a test of cure (ToC) evaluation was performed, with clinical evaluation and microbiological assessments. If clinically indicated, patients were evaluated again at 28 to 35 days after discontinuation of therapy, for assessment of clinical relapse, clinical worsening or need for further treatment.
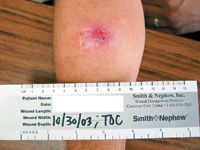
Thirty men and 10 women, with a mean age of 44.7 years (range: 18 to 82 years), were enrolled in the study. Diagnoses were abscess (26 patients), wound (nine patients),and cellulitis (five patients), with involvement of the fascial plane (16 patients), muscle (13 patients), and subcutaneous tissue (11 patients). The majority of infections were community-acquired.
All completedAll patients completed the minimal seven days of treatment and were included in the intent-to-treat (ITT) population.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











