- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Oleogel-S10 for Epidermolysis Bullosa Wounds Demonstrated Accelerated Healing
Author(s):
Phase 3 data of Oleogel-S10 was presented in a late-breaking session at AAD 2023 in New Orleans.
Photo courtesy of the Centers for Disease Control and Prevention
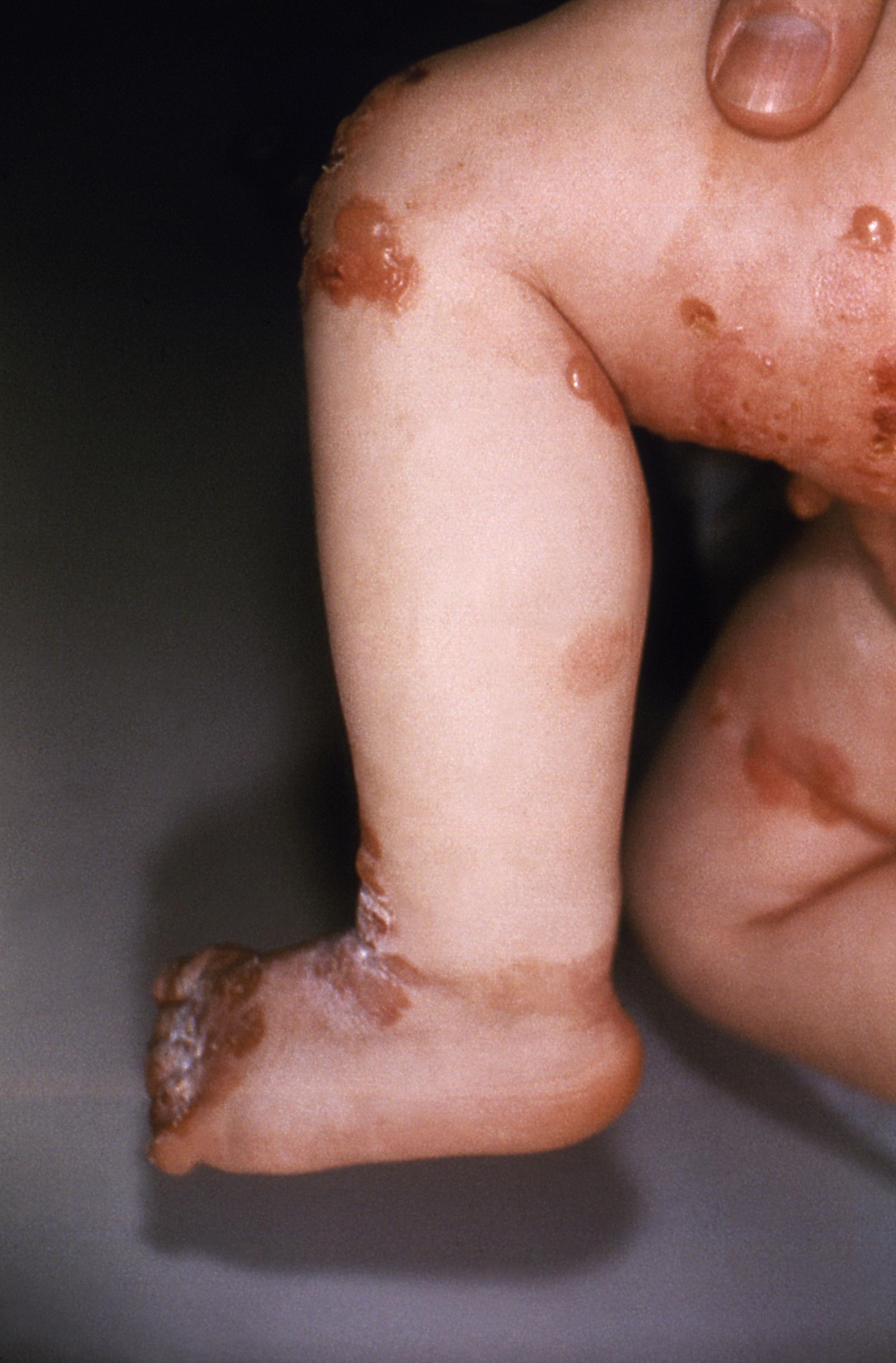
Oleogel-S10 (Amryt Pharma) is the first therapy to demonstrate accelerated healing in epidermolysis bullosa (EB) wounds, according to a late-breaking session at the 2023 American Academy of Dermatology (AAD) Annual Meeting, presented by Dedee Murrell, MD, FAAD, in New Orleans, Louisiana. Murrell, professor of medicine & health at the University of New South Wales Sydney, reviewed the long-term safety and efficacy of Oleogel-S10 from the 24-month open-label phase (OLP) 3 EASE study.
Murrel and colleagues reported safety and total wound burden results from the 24-month OLP, where all patients received treatment with Oleogel-S10. EB Disease Activity and Scarring Index (EBDASI) and Body Surface Area Percentage (BSAP) data were reported without visit windows to reflect a real-world situation more accurately, specifically related to the COVID-19 pandemic. The patient population was made up of dystrophic EB (n=178, 86.8%) and junctional EB (n=25, 12.2%). 141 patients (68.8%) completed the OLP. The mean treatment duration for all patients was 584.7 (246.1) days.
According to Murrell, “Adverse events were reported in 77.1% of all patients in the OLP versus 81.7% of those receiving Oleogel-S10 in the DBP. Mean BSAP for patients treated with Oleogel-S10 in the DBP reduced from 12.1% at study entry to 6.1% with 27 months of treatment. Similarly, the mean EBDASI skin activity score in the Oleogel-S10 group improved from 19.6 to 15.1 after 27 months. In addition, reductions in both BSAP and EBDASI from OLP baseline were observed in patients who transitioned from control gel to Oleogel-S10 in the OLP.”
During the 90-day double-blind phase of the phase 3 EASE, Oleogel-S10 demonstrated significant wound healing compared to vehicle.
“These data support a reassuring long-term safety profile of Oleogel-S10. Furthermore, the reduction in wound burden previously reported with 15 months of Oleogel-S10 treatment is maintained to the end of OLP. This is encouraging given the nature of this chronic genetic disorder in which there is regular cycling of patients’ fragile wounds,” Murrell concluded.
Last October, phase 3 EASE results from Amryt Pharma's (NCT03068780) trial of Oleogel-S10 gel for the treatment of EB were published in the British Journal of Dermatology. EASE is the largest phase 3, randomized, controlled study evaluating treatment options for EB. Currently, Oleogel-S10 is approved in the European Union and Great Britain for the treatment of partial thickness wounds associated with junctional and dystrophic EB in patients aged 6 months and older.
Reference
- Murrel D. Long-term safety and efficacy of Oleogel-S10 (birch triterpenes) in the treatment of epidermolysis bullosa wounds: Results from the 24-month open-label study of the EASE study. Presented at the Late-Breaking Research: Session 2 at the 2023 American Academy of Dermatology Annual Meeting; March 17-21, 2023; New Orleans, LA.
- Photo credits: Centers for Disease Control and Prevention. Public Health Image Library. https://phil.cdc.gov/Details.aspx?pid=14548
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







