- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Oral Ivarmacitinib Demonstrates Significant Atopic Dermatitis Improvement
Author(s):
Results from the phase 3 study were presented at an AAD late-breaking research session.
Oral ivarmacitinib demonstrated statistically significant improvement in patients with moderate-to-severe atopic dermatitis (AD), according to new research presented by Yan Zhao, MD, in a late-breaking research session at the 2023 American Academy of Dermatology (AAD) Meeting in New Orleans, LA.1
Ksenia Kirillovykh/AdobeStock
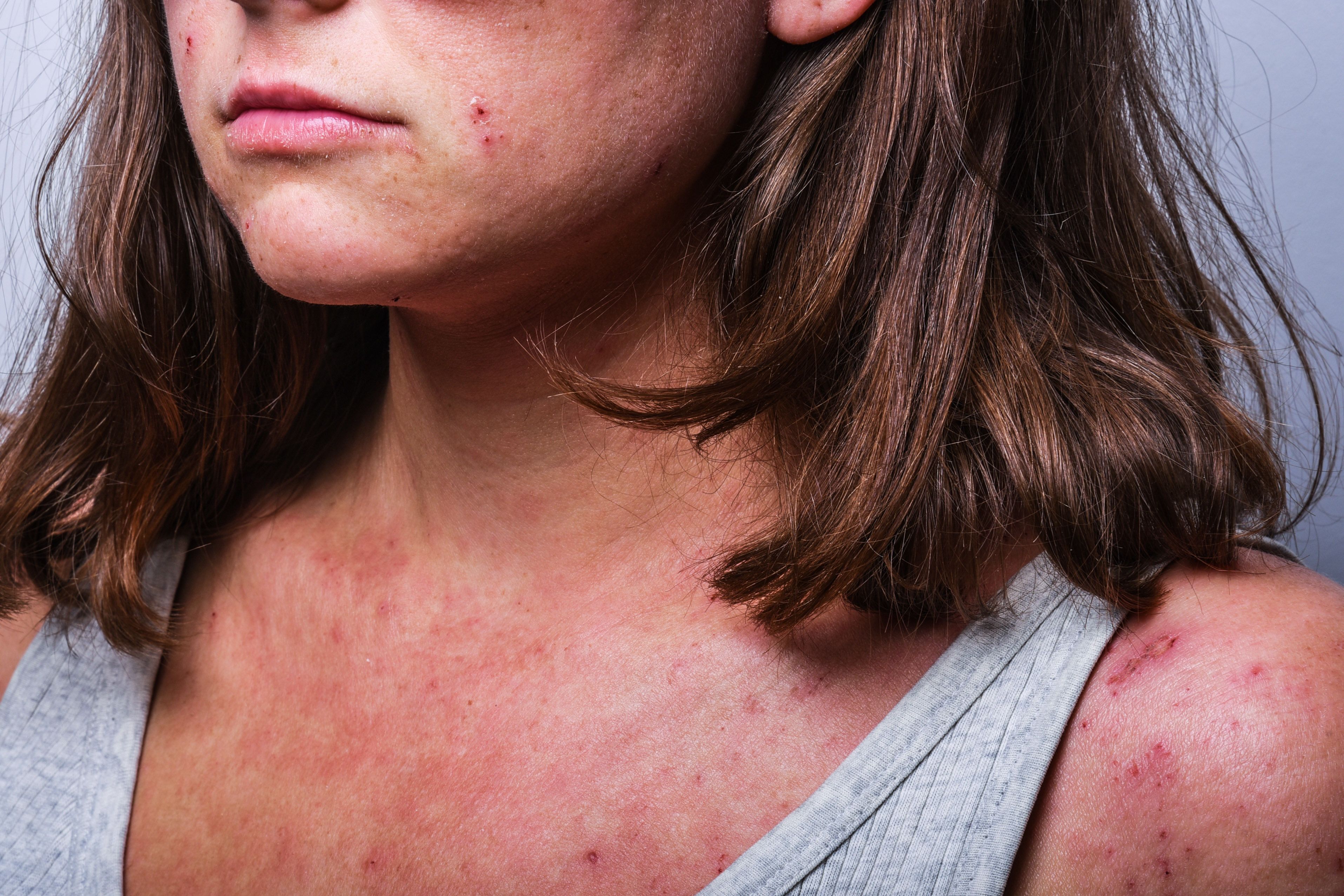
Nearly 2.4% of the world’s population is impacted by AD. Ivarmacitinib, a JAK1 inhibitor, is under clinical development for several other conditions, including alopecia areata, ankylosing spondylitis, Crohn’s disease, rheumatoid arthritis, and ulcerative colitis.
The phase 3, randomized trial was conducted in China and Canada over a duration of 52 weeks. Researchers sought to determine the safety and efficacy of oral ivarmacitinib in patients with AD.
In the first 5 weeks, 467 prospective participants were screened. Most participants were adolescents and adults with moderate-to-severe AD ranging from 12 to 75 years of age. All participants were required to have met Hanafin and Rajka AD diagnostic criteria for at least 1 year prior to the start of the study.
Researchers also sought participants who had inadequate responses to topical corticosteroids or calcineurin inhibitors for 6 months prior to enrolling in the study, or who required systemic therapy. Ideal AD scoring requirements included:
- Investigator Global Assessment (IGA) scale: 3-4
- Eczema Area and Severity Index (EASI): Greater than or equal to 16
- Worst Itching Intensity Numerical Rating Scale (WI-NRS): Greater than or equal to 4
- Body Surface Area: Greater than or equal to 10
Ultimately, 336 patients were randomized following the screenings. Participants were assigned to be treated with ivarmacitinib 8 mg QD (n=112), ivarmacitinib 4 mg QD (n=113), or a placebo (n=111). Of all participants, 17% were adolescents.
In order to measure for efficacy, researchers established the following endpoints:
- CO-PRIMARY ENDPOINTS: IGA0/1 and EASI-75 responses by week 16
- SECONDARY EFFICACY ENDPOINTS: WI-NRS4, EASI50, and EASI90 responses; SCORing Atopic Dermatitis (SCORAD), Dermatology Life Quality Index (DLQI), and Patient Oriented Eczema Measure (POEM) scale scoring
Participants were asked to use their assigned treatments for a core treatment period of 16 weeks.
At week 16, participants’ IGA and EASI scores were statistically significantly higher in the ivarmacitinib treatment groups than they were in the placebo group. The IGA response rate at week 16 was 42% for the 8 mg group, 36% for the 4 mg group, and 9% for the placebo group, while the EASI response rate at week 16 was 66% for the 8 mg group, 54% for the 4 mg group, and 22% in the placebo group.
Participants continued using ivarmacitinib treatment from week 16 until week 52. Additionally, researchers conducted a 4-week follow-up.
In total, 12 participants across all 3 treatment groups discontinued treatment due to adverse events (AE). Common treatment emergent AEs included upper respiratory tract infections, folliculitis, and increased blood creatine phosphokinase.
"Both ivarmacitinib doses were well-tolerated with no increase in the incidence of serious adverse events and adverse events leading to discontinuation of study drug compared with placebo,” the study said. “The totality of ivarmacitinib efficacy and safety data demonstrates a favorable benefit-risk profile for 8 mg and 4 mg once daily dose in treating patients with moderate to severe atopic dermatitis.”
Conflict of Interest Disclosures
- Primary investigator of the study
- Clinical Advisor to Reistone Biopharma Co., Ltd.
Reference
- Zhao Y. Ivarmacitinib, a highly selective JAK1 inhibitor for moderate-to-severe atopic dermatitis: 16-week results from a phase 3 pivotal study (QUARTZ3). Presented at the 2023 American Academy of Dermatology Annual Meeting. March 17-21, 2023; New Orleans, Louisiana.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











