- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
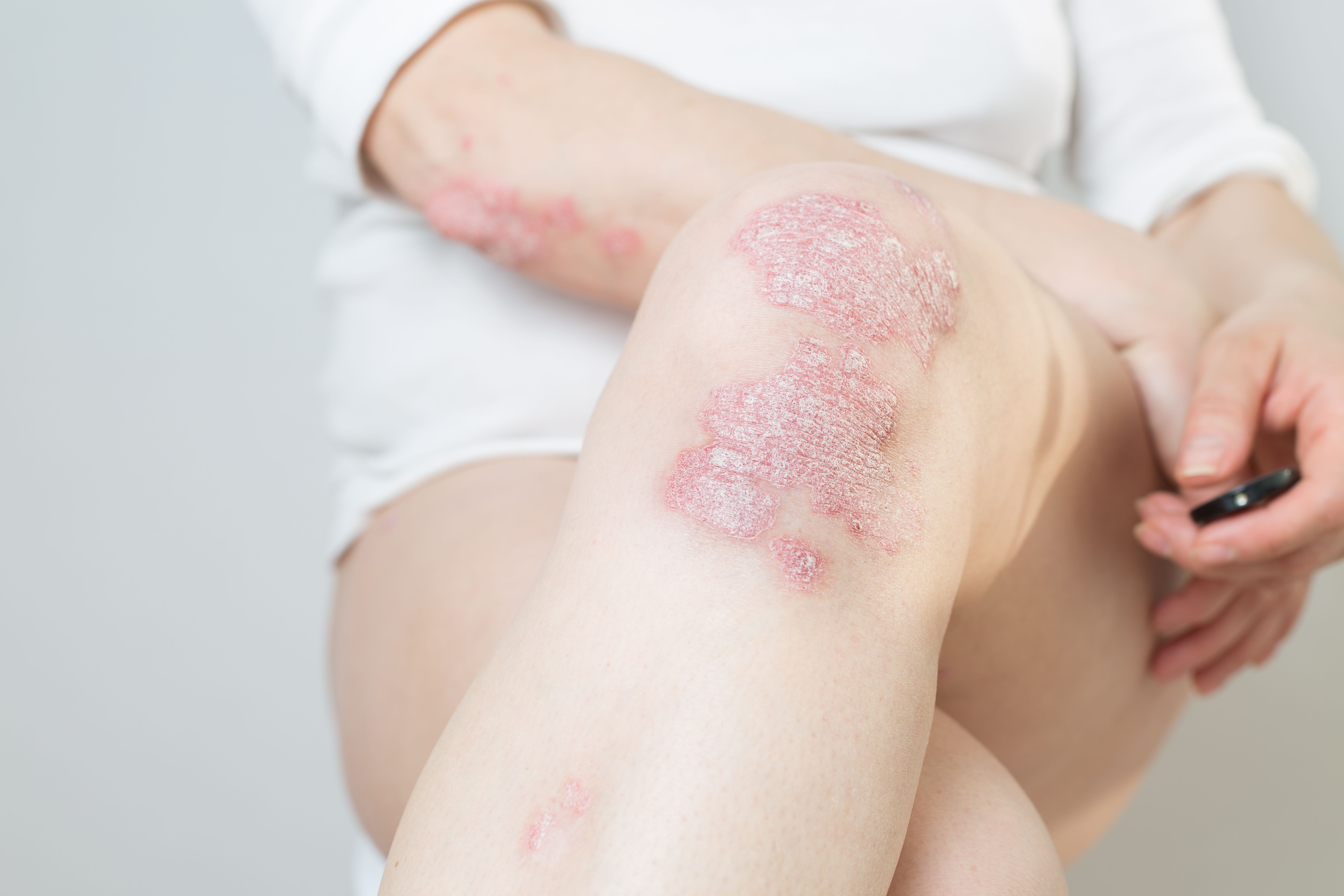
Oral Treatment of Plaque Psoriasis Achieves Primary and Secondary Endpoints in Trial
Author(s):
Janssen Pharmaceutical Companies announced its oral treatment for moderate to severe plaque psoriasis will be taken to Phase 3 clinical trials.
Janssen Pharmaceutical Companies of Johnson & Johnson’s interleukin-23 receptor (IL-23R) antagonist peptide JNJ-2113 met its primary and secondary endpoints in a Phase2b FRONTIER 1 clinical trial of adults with moderate to severe plaque psoriasis the company recently announced.1
JNJ-2113 is the first and only oral IL-23R for treatment of patients with moderate to severe plaque psoriasis. At a dose of 100 mg twice a day, 78.6% (n=42) of participants reached the primary endpoint of 75 percent improvement of skin lesions as measured by the Psoriasis Area and Severity Index (PASI) at 16 weeks compared to 9.3% of participants taking a placebo.
Of those participants receiving 100 mg of JNJ-2113 twice a day, 59.5% achieved PASI 90 compared to 2.3% of those taking a placebo.
PASI 100 was reached by 40.5% of participants taking JNJ-2113 twice a day compared with 0% of those taking a placebo.
JNJ-2113 works by binding with high compatibility to the IL-23R and has properties that enable it to be absorbed when taken orally. It selectively and effectively blocks IL-23 signaling and downstream inflammatory cytokine production.
"The development of a novel oral therapy that specifically targets IL-23R could potentially change the treatment paradigm for patients living with moderate-to-severe plaque psoriasis," said Lloyd Miller, MD, PhD, Vice President, Immunodermatology Disease Area Stronghold Leader, Janssen Research & Development, LLC, in a press release. "Until now, advanced psoriasis treatments have been largely limited to injectable biologics. An oral therapy that can uniquely inhibit the IL-23 pathway by directly targeting the IL-23 receptor could help address the needs and preferences of patients, and may offer greater freedom, with the aim of driving greater adoption of advanced treatment."
Participants reporting adverse events (AEs) were similar between those taking JNJ-2113 and those taking a placebo (52.4% versus 51.2% respectively). The most common AEs were infections including COVID-19, nasopharyngitis, and upper respiratory tract infection.
Shares of Protagonist Therapeutics initially fell after the results were released showing that JNJ-2113 was not as effective as injectable psoriasis treatments. However, after Janssen reported that the trials did reach primary and secondary endpoints, shares regained some of their value.
Based on the positive results, the company plans to take JNJ-2113 to Phase 3 clinical trials.
Reference
- Janssen announces positive topline results for JNJ-2113 – a novel, first and only oral IL-23 receptor antagonist peptide in development for moderate-to-severe plaque psoriasis. News Release. July 4, 2023. Accessed July 6, 2023. https://www.prnewswire.com/news-releases/janssen-announces-positive-topline-results-for-jnj-2113a-novel-first-and-only-oral-il-23-receptor-antagonist-peptide-in-development-for-moderate-to-severe-plaque-psoriasis-301869349.html