- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Oxymetazoline Efficacious in Reducing Rosacea-Associated Facial Erythema
Author(s):
When compared to a vehicle treatment, patients with rosacea being treated with oxymetazoline had a more significant reduction facial erythema.
In patients with rosacea-associated facial erythema, oxymetazoline proved to be more efficacious in reducing redness than vehicle treatment.
Siniehina/AdobeStock
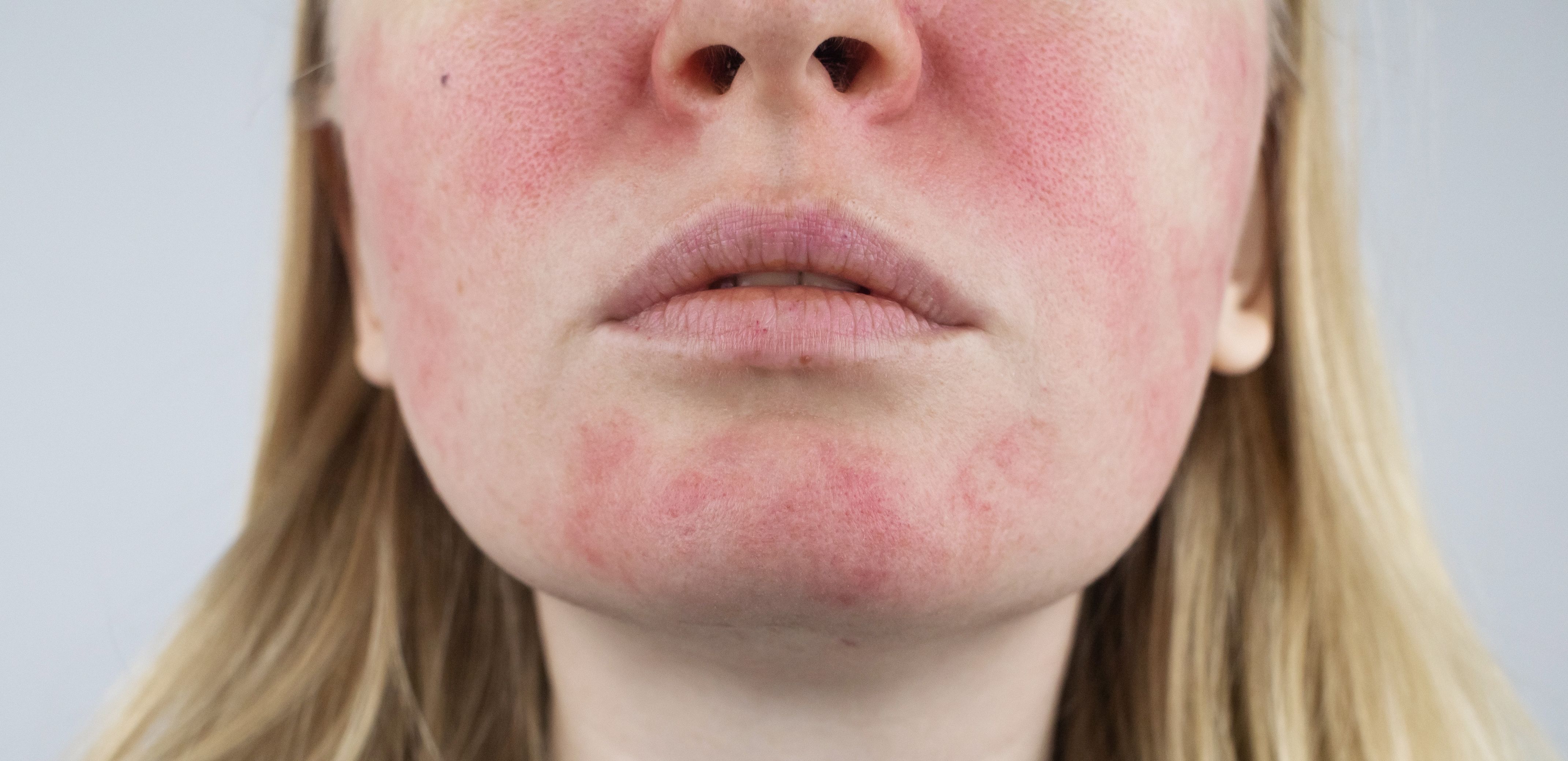
In a recent meta-analysis,1 researchers sought to investigate the drug’s overall safety and efficacy, citing a lack of conclusion or consensus on the effectiveness and adverse events stemming from treatment. Currently, the α1-andrenergic receptor agonist is approved for the treatment of persistent facial erythema associated with rosacea in adult populations.2
Researchers used databases such as Pubmed, Embase, and Cochrane Library from the time of their respective establishment to May 2021, using key search terms such as, “acne rosacea,” “erythematotelangiectatic rosacea,” “granulomatous rosacea,” “ocular rosacea,” “oxymetazoline,” “papulopustular rosacea,” “phymatous rosacea,” and “rosacea.”
The literature search and subsequent screenings and data extractions were completed by 2 independent researchers, though in cases of disagreement, a third researcher was brought in to achieve consensus. Papers included in the meta-analysis were randomized controlled trials limited to English wherein eligible patients were assigned on a random basis to be treated either with oxymetazoline hydrochloride 1.0% or a vehicle treatment.
Prospective papers were excluded from analysis if they were systematic reviews, duplicate publications, provided research without full text, contained incomplete information, involved animals, or if researchers were unable to conduct data extraction.
Researchers extracted all relevant data for each paper, including study author, year, study area, research type, number of cases, and disease-specific indicators, such as Clinician Erythema Assessment (CEA) success rate, Subject Self-Assessment for rosacea facial redness (SSA) success rate, satisfaction rate, and rate of treatment-emergent adverse events (TEAEs).
All papers were evaluated for quality through blind assessment, risk assessment, random sequence generation, allocation concealment, blind methodology, blind evaluation, and result data completeness.
After extracting studies that met the aforementioned criteria, researchers had pulled 7 studies for complete quantitative synthesis. This included a total of 3934 patients, 2056 of which were treated with oxymetazoline, and 1869 of which were treated with a vehicle.
As a result, researchers found significantly higher CEA and SSA success and improvements in patients being treated with oxymetazoline. However, patients being treated with oxymetazoline reported significantly higher incidence of TEAEs than their vehicle group counterparts. Researchers found, though, that the only TEAE reported in this patient group that was significantly higher than in the vehicle group was application-site dermatitis. Other TEAEs, such as worsening inflammatory lesions of rosacea, application-site pruritus, application-site papules, headache, upper respiratory tract infections, nasopharyngitis, and vomiting, did not have significant differences in incidence rates between treatment groups.
Researchers indicated that potential limitations included size of the sample and a potential lack of objectivity, having an impact on the investigation of low bias credibility.
“Oxymetazoline is effective and can be selected for the treatment of persistent facial erythema of rosacea,” study authors wrote. “Additionally, application-site dermatitis was the most important one. Additionally, that most of the TEAEs of oxymetazoline were considered mild or moderate in severity, of which application-site dermatitis was the most important one.”
References
- Liu F, Zhou Q, Wang H, Fu H, et al. Efficacy and safety of oxymetazoline for the treatment of rosacea: a meta-analysis. J Cosm Dermatol. https://doi.org/10.1111/jocd.15747
- plc A. Allergan announces FDA approval of RhofadeTM (Oxymetazoline Hydrochloride) cream, 1% for the topical treatment of persistent facial erythema associated with rosacea in adults. PR Newswire: press release distribution, targeting, monitoring and marketing. June 26, 2018. Accessed May 10, 2023. https://www.prnewswire.com/news-releases/allergan-announces-fda-approval-of-rhofade-oxymetazoline-hydrochloride-cream-1-for-the-topical-treatment-of-persistent-facial-erythema-associated-with-rosacea-in-adults-300393385.html.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














