- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Pediatric dermatoses: Off-label biologic use carries risks, benefits
Author(s):
Psoriasis in adults is among the most-researched inflammatory skin diseases. Yet treatment options for moderate-to-severe atopic dermatitis and psoriasis in children represents a largely unmet medical need, according to an expert.

Key Points

"There are no age- and indication-specific FDA (Food and Drug Administration)-approved drugs or biologic agents to treat children with these diseases, but there are many disincentives to collecting the necessary data in this population," she says.
Negative impact
According to Dr. Siegfried, other companies such as Centocor (ustekinumab, Stelara) and Abbott Laboratories (adalimumab, Humira) are not pursuing pediatric approval with any urgency.
"Ironically, Amgen sponsored the largest placebo-controlled, prospective, multicenter, double-blind trial of any systemic agent used to treat children with an inflammatory skin disease," Dr. Siegfried says. "But despite the highest level of evidence, we still don't have FDA approval. The negative impact for these vulnerable patients is clinical and financial."
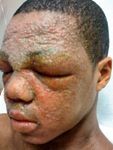
As a result, clinicians who care for these children will have to choose between recommending suboptimal treatments, or bearing all the responsibility for prescribing non-evidence-based, off-label therapy.
Available treatments
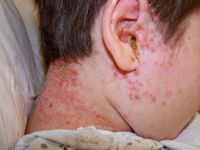
A few biologic agents have been used off-label to treat pediatric psoriasis and atopic dermatitis, the two major pediatric chronic inflammatory skin diseases.
"While psoriasis is a disease mostly of adults, with a peak age of onset of at 18 or 20 years, atopic dermatitis affects 20 percent of children, with a significant number suffering from the moderate-to-severe form of the condition," Dr. Siegfried says. "In my practice, I see kids with moderate-to-severe psoriasis, but for every one with psoriasis, I see 10 with moderate-to-severe atopic dermatitis.
"My personal experience mirrors epidemiologic data that has revealed the overwhelming prevalence of atopic dermatitis," she adds. "But because it is primarily a disease of children, new drug development has been hampered by several obstacles."
The efficacy of anti-tumor necrosis factor drugs, including Enbrel, Humira and Remicade (infliximab, Centocor), has been well-established for psoriasis.
"These are drugs that not only don't make atopic conditions better, but they may trigger atopy, as evidenced by anecdotal observations. Raptiva (efalizumab, Genentech) which was studied in adults, was one of the drugs that appeared to have the potential to make atopic dermatitis better, but it is no longer available," she says.
Further research with this drug for atopic dermatitis is unlikely, Dr. Siegfried says. Astellas Pharma experienced significant regulatory scrutiny with its drug Protopic (tacrolimus), which now carries a "black box" warning.
Current biologics used off-label to treat atopic dermatitis include interferon gamma (Actimmune, InterMune), and intravenous immunoglobulin (IVIg).
Interferon gamma, a daily subcutaneous injection administered two to five days a week, costs about $5,000 a month. It was studied in small clinical trials more than a decade ago for atopic dermatitis. The majority of subjects were adults and the results were not promising enough to pursue FDA approval for this indication. However, like Enbrel, the drug may be more effective in children.
IVIg is another extraordinarily expensive drug and requires monthly half-day outpatient infusions. Small studies for atopic dermatitis more than a decade ago showed promise for some children, Dr. Siegfried says.
Potentiating the disease
The highest level of evidence for treating severe inflammatory skin disease in children supports the use of systemic immunosuppressive agents, such as cyclosporine, azathioprine, mycophenolate mofetil and methotrexate, which have significant safety concerns.
Furthermore, when the immunosuppressive drugs are used to treat atopic dermatitis patients, as subset of children develop severe intermittent flares associated with secondary or occult infections such as herpes, widespread molluscum, recurrent furunculosis or sinusitis.
"I carefully screen these children for evidence of infection prior to initiating immunosuppressive therapy," Dr. Siegfried says. "If there is evidence of infection or a history of eczema herpeticum, I recommend interferon gamma or IVIg, rather than methotrexate, cyclosporine or azathioprine. We don't expect much new data on these drugs."
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.