- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Phase 2a Study Demonstrates Dose-Dependent Tolerability of Oral Orismilast in Hidradenitis Suppurativa
Author(s):
Results of this study may also be indicative of oral orismilast's ability to contribute to meaningful clinical improvements, investigators noted.
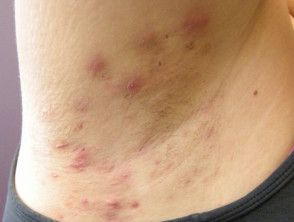
In a recent phase 2a study, investigators found that oral orismilast demonstrated dose-dependent tolerability in patients with hidradenitis suppurativa (HS). Furthermore, the results of this study may also be indicative of the PDE4 inhibitor's ability to contribute to meaningful clinical improvements of HS.
Study authors Frederiksen et al sought to explore the safety, tolerability, and efficacy of an oral dose of orismilast 10 mg to 40 mg in patients with HS. They cited the growing role of PDE4 inhibition in HS management, noting that orismilast may potentially possess improved efficacy when compared to other PDEF4 inhibitors currently available.
According to study authors, this study marks the first treatment experience of orismilast among patients with HS.
The single center, single arm, and open-label study, located in Denmark, enrolled adult patients with mild to severe HS between October 2021 and December 2022. HS diagnosis was defined by an International HS Severity score (IHS4).
Patients eligible for inclusion in the study were also required to have an IHS4 score of equal to or greater than 2, a total number of abscesses and nodules equal to or greater than 2, and a draining tunnel count of equal to or less than 30.
During a 4-week screening period, 26 patients were screened. Upon the conclusion of the screening, 20 patients were ultimately enrolled, thus receiving at least 1 dose of oral orismilast. Of the 20 patients involved in the study, 4 had mild HS, 8 had moderate HS, and 8 had severe HS.
In total, 9 patients completed the 16 weeks of treatment involved in the study. Eleven patients in total discontinued the trial prior to the 16 week mark, with 6 discontinuing due to drug-related adverse events (AEs), non-IMP-related AEs, or other reasons (specified as lost to follow-up or personal reasons).
Among the 9 patients who completed the trial, the average total count of abscesses and nodules was 5.33 [1.27; 9.40] and the average total lesion count was 8.78 [3.51; 14.04].
Investigators initially aimed for a target dose of 40 mg given twice daily by week 2. However, rapid dose escalation was discontinued and altered to involve a more individualized approach with a slower titration and lowered end dose.
From baseline to conclusion of the study, patients completing the study achieved a 33.1% improvement in total abscess and nodule count, with a reduction from 5.3 [95% CI: 1.3; 9.4] to 3.8 [95% CI: −2.5; 10.0]. This patient group also achieved a 41.86% change in average total lesion count, and a 43.78% reduction in mean IHS4 score.
"In contrast to changes in the [abscesses and nodules]-count, changes in the total lesion count, the IHS4-score and the [Hidradenitis Suppurativa Clinical Response] also encompasses assessment of draining tunnels, which may provide a more thorough assessment of the changes seen in the patients," investigators wrote.
Also reducing from baseline to conclusion was the average global pain score.
Throughout the 16 week trial period, 197 AEs were reported, predominantly including adverse drug reactions such as diarrhea or loose stool, abdominal pain, nausea, vomiting, headache, and dizziness. The majority of AEs were mild in nature, and most were reported at or before the 4-week mark.
However, it is reported that none of the AEs deemed to be serious in nature were related to orismilast.
"Clinically relevant improvements occurred during orismilast treatment of patients with HS," wrote Frederiksen et al. "The results of this small trial suggest that PDE4B/D-inhibition with orismilast may lead to clinical improvements in HS. However, larger trials at the most tolerable dose ranges are warranted based on the results of this exploratory trial. The safety profile was similar to what was seen with orismilast in psoriasis, and for other PDE4-inhibitors."
Reference
Frederiksen CG, Sedeh FB, Taudorf EH, Saunte DM, Jemec GBE. Orismilast for the treatment of mild to severe hidradenitis suppurativa: week 16 data from OSIRIS, a phase 2a, open-label, single-centre, single-arm, dose-finding clinical trial. December 26, 2023. Accessed January 2, 2024. https://onlinelibrary.wiley.com/doi/pdfdirect/10.1111/jdv.19770.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.