- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Phase 3 results demonstrate dupilumab efficacy
Author(s):
New data suggests positive safety and efficacy profile. Further studies need to assess long-term use.
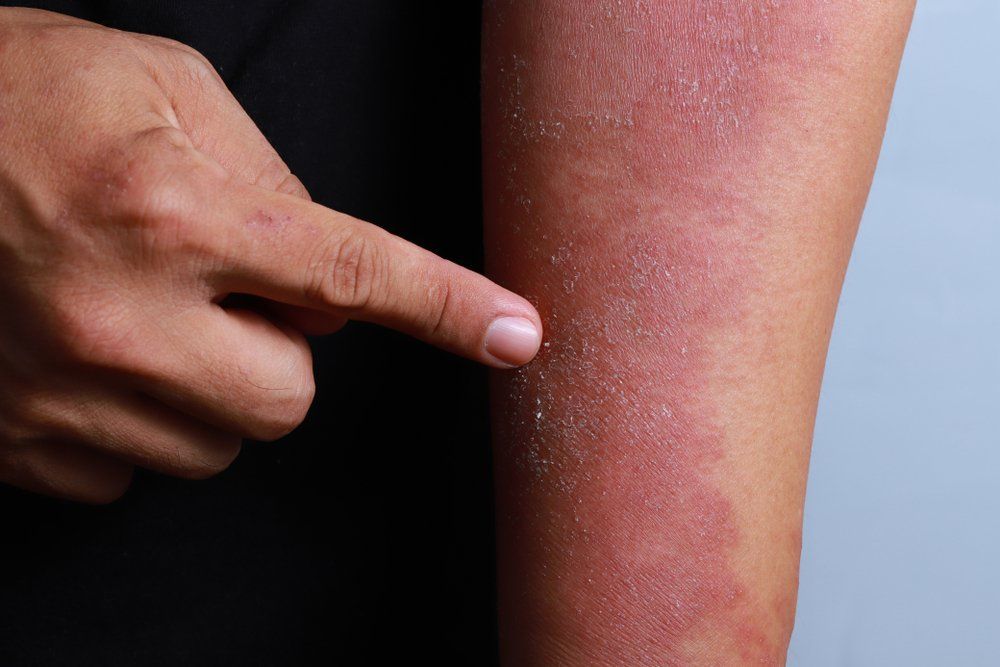
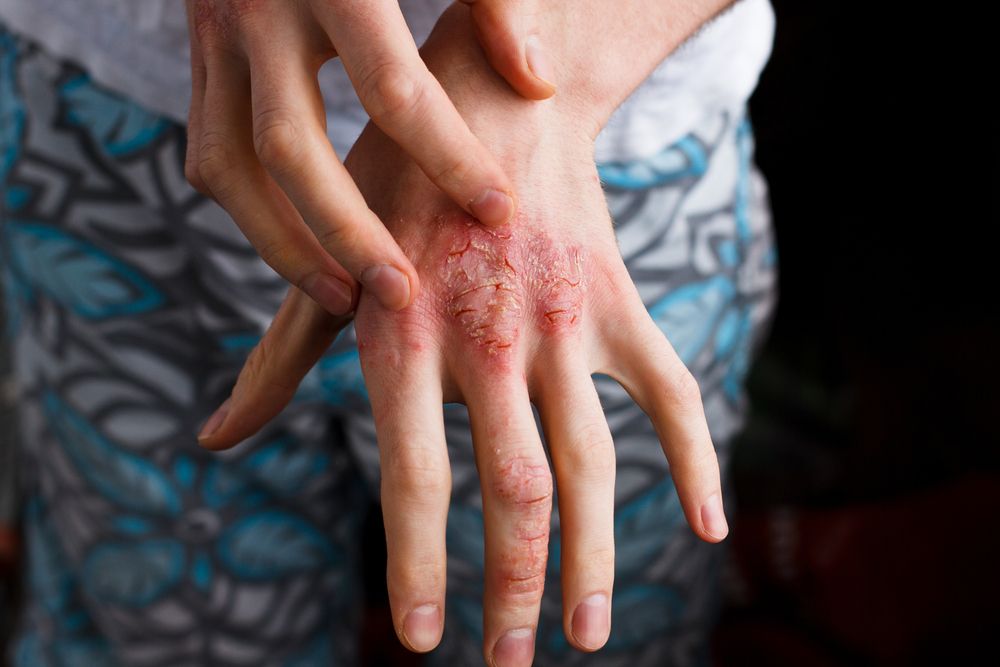
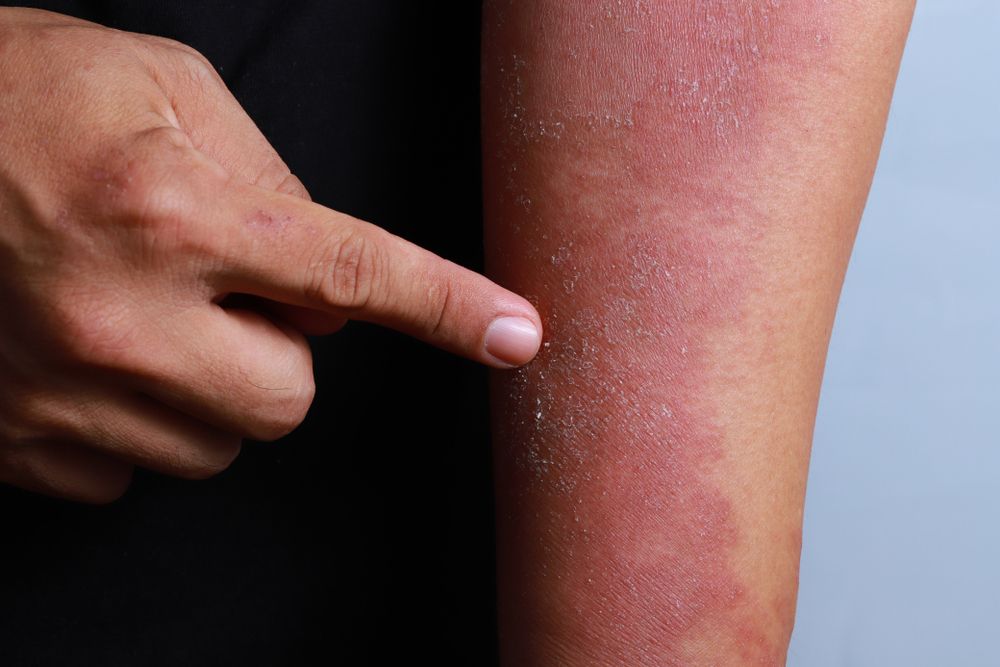
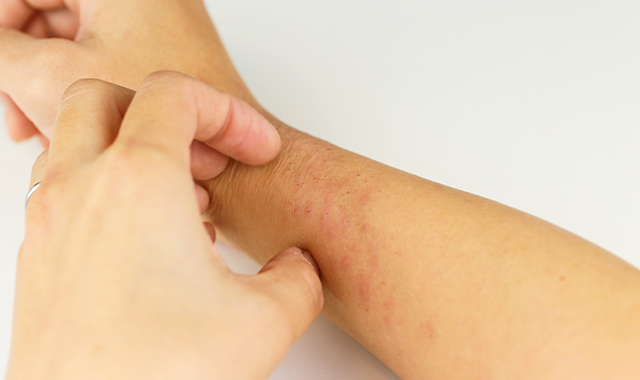
For adults with moderate-to-severe eczema, there are positive phase 3 outcomes suggesting dupilumab could offer a much-needed safe, effective future treatment option, according to a study.
Dupilumab improved AD, including associated pruritus, anxiety, depression and quality-of-life, compared to placebo in adults with moderate-to-severe disease, according to a paper on two phase 3 trials published online September 30 in the New England Journal of Medicine.
The research, funded by Sanofi and Regeneron Pharmaceuticals, shows there is a promising new option for patients whose quality of life was severely diminished by their disease, says the study’s lead author Eric Simpson, M.D., M.C.R., who directs the clinical studies unit in Oregon Health and Science University’ (OHSU’s) dermatology department.
“Additional clinical trials are needed to explore whether long-term use of dupilumab is safe, but it represents a potential new approach for our patients who have suffered without good options for far too long,” he says in an OHSU press release.
Dr. Guttman-Yassky, who is also an author on this study, agrees that there are no good options for adults with moderate-to-severe AD.
"We have oral prednisone [which is approved for the indication], but I never give it to patients because it has a deleterious disease rebound--after you start the oral prednisone, everything comes back with a vengeance," Dr. Guttman-Yassky says.
Cyclosporin is approved for psoriasis, and dermatologists often prescribe it for eczema, but it's not approved for eczema. Cyclosporin is an immune suppressant, with very worrisome side effects with as little as one year of use, according to Dr. Guttman-Yassky.
"There is phototherapy, which is a very safe treatment, but there are few patients who can accommodate it in their schedules. You have to come two to three times a week to the doctor’s office," she says.
Dupilumab, a human anti-interleukin (IL)-4 receptor-alpha monoclonal antibody, which inhibits IL-4 and IL-13 signaling that may be fueling atopic and allergic diseases.
The 16-week, randomized phase 3 trials involved one group of 671 participants and a second group of 708 subjects. All were 18 years or older and had moderate-to-severe AD that had not responded to existing treatments or who were not candidates for existing therapies.
Participants received either a weekly dupilumab 300 mg dose; a 300 mg dose of the medication every other week; or placebo injections.
Researchers found in first study (SOLO 1) that 38% of those who received dupilumab every other week saw a clearing or near clearing of their AD skin lesions, equaling a two or more point reduction from baseline on Investigator’s Global Assessment scoring. In the weekly dupilumab group, 37% of subjects achieved clearing or near clearing. And one in 10 in the placebo group achieved clearing or near clearing of their eczema.
The second study, SOLO 2, had similar results: 36% of participants who received the treatment every other week, as well as those receiving it weekly, experiencing a complete clearing or near clearing, versus 8% of those who received a placebo.
Those taking dupilumab also experienced improvements in pruritus, symptoms of anxiety and depression and quality of life.
The drug’s safety profile was good, with injection-site reactions and conjunctivitis being more frequent in the active arms. The two deaths reported among subjects taking dupilumab were determined to be unrelated to the drug, but more studies are underway to determine the medication’s long-term safety profile.
"We did not see increased skin infections in these patients, and, in my own study patients, I didn't notice any significant safety issues," Dr. Guttman-Yassky says. "I can tell you from my own patients in the study that they were very happy. They had a very poor quality of life before the study, with eczema affecting every part of their bodies…. Many of them were facing divorces. I heard from many patients that they considered suicide due to the deleterious effects of the disease, including lack of sleep and constant itching. They didn't know what to do. Now they have good quality-of-life and can resume their normal activities. They work. They enjoy their lives."
Dr. Guttman-Yassky says dupilumab could receive FDA approval by the end of March 2017-maybe even before.
“The FDA gave the drug breakthrough status,” she says. “And [if approved] this will be a new era for atopic dermatitis patients.”
Trials of longer duration are needed to assess the long-term effectiveness and safety of dupilumab.
Disclosures: Dr. Simpson has been an investigator and consultant for Sanofi and Regeneron Pharmaceuticals.
Dr. Guttman-Yassky is a board member for Sanofi Aventis, Regeneron and has received consultancy fees from Regeneron and Sanofi. She is also a board member of Stiefel/GlaxoSmithKline, MedImmune, Celgene, Anacor, AnaptysBio, Celsus, Dermira, Galderma, Glenmark, Novartis, Pfizer, Vitae and Leo Pharma; has received consultancy fees from MedImmune, Celgene, Stiefel/GlaxoSmithKline, Celsus, BMS, Amgen, Drais, AbbVie, Anacor, AnaptysBio, Dermira, Galderma, Glenmark, LEO Pharma, Novartis, Pfizer, Vitae, Mitsubishi Tanabe and Eli Lilly; and has received research support from Janssen, Regeneron, Celgene, BMS, Novartis, Merck, LEO Pharma and Dermira.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.