- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Positive Long-Term Results for Roflumilast Cream in Young Children
Author(s):
Results from the study highlight that the therapy improves AD symptoms over time, with 71.9% of participants achieving substantial improvement.
Image Credit: © Марина Терехова - stock.adobe.com
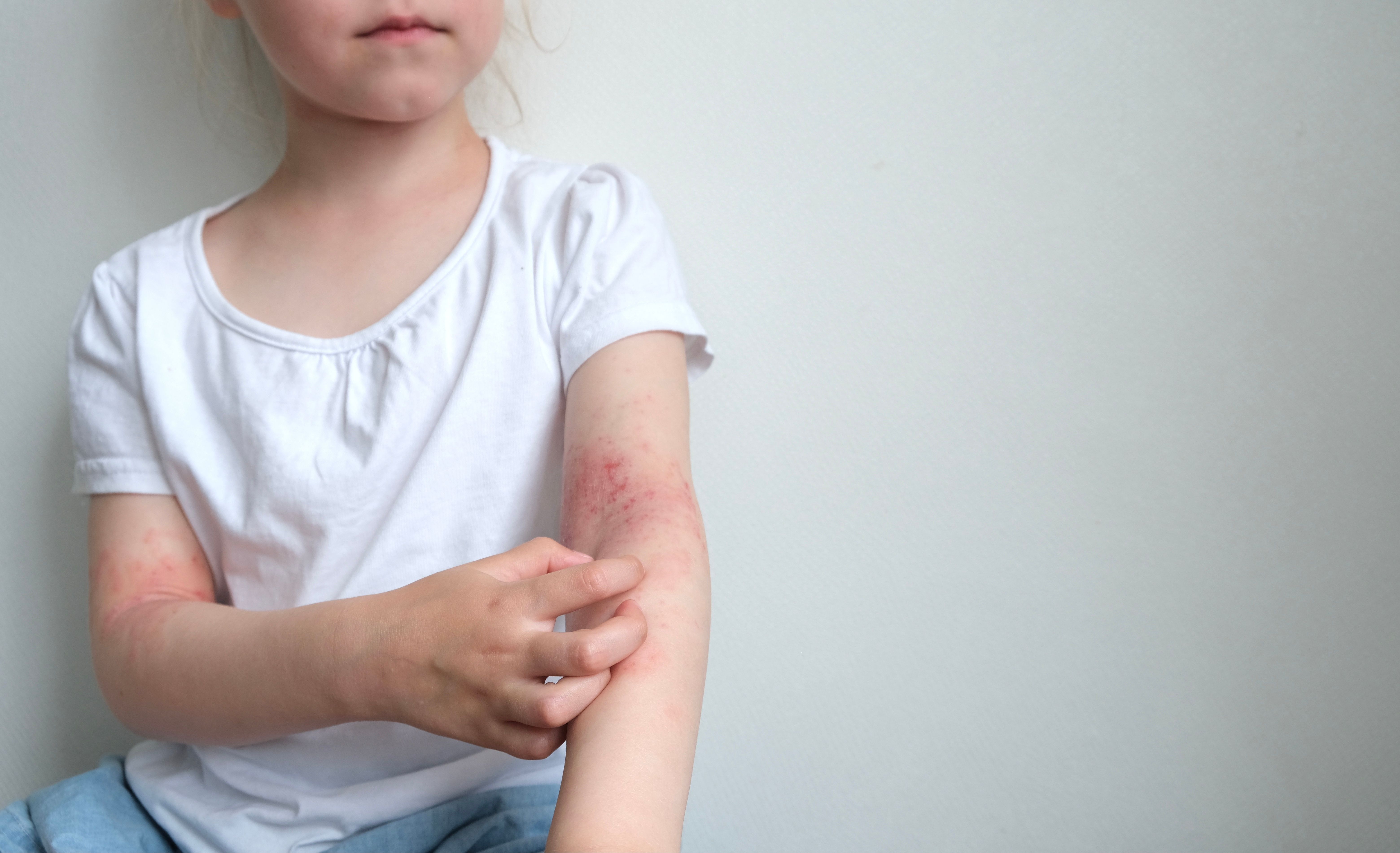
Arcutis Biotherapeutics announced today promising results from the INTEGUMENT-OLE study, an extended open-label trial evaluating the long-term use of roflumilast cream 0.05% for treating mild to moderate atopic dermatitis (AD) in children aged 2 to 5 years.1 This study builds on previous findings from the phase 3 INTEGUMENT-PED trial, highlighting the cream's durable efficacy and favorable safety profile.2
“Roflumilast cream is uniquely formulated with the AD patient in mind, to deliver treatment without sensitizing excipients and irritants, which can often disrupt the skin barrier. We are excited by these results, which reinforce the strength of our ZORYVE product portfolio and specifically demonstrate the long-term efficacy, safety and tolerability profile of our once-daily roflumilast cream for the treatment of pediatric AD,” said Patrick Burnett, MD, PhD, FAAD, chief medical officer at Arcutis. “Based on these positive results, we are convinced that, if approved, roflumilast cream 0.05% can help provide immediate as well as long-term management of this burdensome skin condition, expanding the treatment population to children down to age 2.”
The INTEGUMENT-OLE study involved children who had previously participated in the phase 3 trial and continued using roflumilast cream 0.05% for up to 56 weeks. According to the company, the results of the trialdemonstrated that the cream was well-tolerated throughout the study period, with no new safety concerns emerging. They found efficacy not only persisted but improved over time. Specifically, researchers reported 71.9% of participants who continued from the roflumilast treatment arm achieved a 75% improvement from baseline in the Eczema Area and Severity Index (EASI-75) score after 56 weeks. This indicates significant and sustained improvement in the severity of their AD.
“When choosing a therapy for very young children, health care providers and caregivers are looking for treatments that provide both rapid relief and are well-tolerated and suitable for long-term use,” said Adelaide Hebert, MD, professor of dermatology and pediatrics at UTHealth Houston, and INTEGUMENT trial investigator. “These results build upon the findings from the phase 3 trial of roflumilast cream 0.05% that demonstrated rapid efficacy within the first 4 weeks of treatment, and further showed long-term durable efficacy and tolerability of investigational roflumilast cream, with continued improvement over the course of the long-term study.”
Additionally, 53.8% of these participants met the validated Investigator Global Assessment-Atopic Dermatitis (vIGA-AD) success criteria, which is defined as achieving a vIGA-AD score of 0 or 1 along with a 2-grade improvement from baseline. These results suggest that roflumilast cream 0.05% is highly effective in reducing the overall severity of AD in this age group.
The study's safety profile was consistent with earlier findings from the 4-week INTEGUMENT-PED trial. Adverse events were generally mild to moderate, with a low overall incidence. The most frequently reported adverse events included upper respiratory tract infections, nasopharyngitis, pyrexia, influenza, COVID-19, and otitis media. Importantly, only 3.0% of participants discontinued the study due to adverse events, underscoring the cream's tolerability.
Based on these positive results, Arcutis stated it plans to submit a supplemental New Drug Application to the FDA in the first quarter of 2025, seeking approval for the use of roflumilast cream 0.05% in children aged 2 to 5 years. Currently, the related product roflumilast cream 0.15% (Zoryve) is approved for treating mild to moderate AD in adults and pediatric patients aged 6 and older.
Overall, the study reinforces roflumilast cream 0.05% as a promising treatment option for young children with AD, demonstrating both immediate and sustained improvements in disease management.
References
- Arcutis announces positive long-term results of roflumilast cream 0.05% show durable and improved efficacy over time and favorable safety profile in treatment of children aged 2 to 5 with mild to moderate atopic dermatitis. News Release. Arcutis Biotherapeutics. Published August 28, 2024. Accessed August 28, 2024. https://www.arcutis.com/arcutis-announces-positive-long-term-results-of-roflumilast-cream-0-05-show-durable-and-improved-efficacy-over-time-and-favorable-safety-profile-in-treatment-of-children-aged-2-to-5-with-mild-to-moder/
- Arcutis announces positive results from INTEGUMENT-PED pivotal phase 3 trial of roflumilast cream 0.05% for the treatment of atopic dermatitis in children ages 2 to 5. News Release. Arcutis Biotherapeutics. Published September 19, 2023. Accessed August 28, 2024. https://www.arcutis.com/arcutis-announces-positive-results-from-integument-ped-pivotal-phase-3-trial-of-roflumilast-cream-0-05-for-the-treatment-of-atopic-dermatitis-in-children-ages-2-to-5/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.









