- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Positive Phase 2b Results Unveiled in Atopic Dermatitis, Pruritus Treatment
Author(s):
B244 demonstrated significant levels of efficacy in patients with mild-to-severe atopic dermatitis and itch.
B244, a live topical formulation of ammonia oxidizing bacteria, demonstrated significant efficacy in patients with mild-to-severe atopic dermatitis (AD) and pruritus.
Ольга Тернавская/AdobeStock
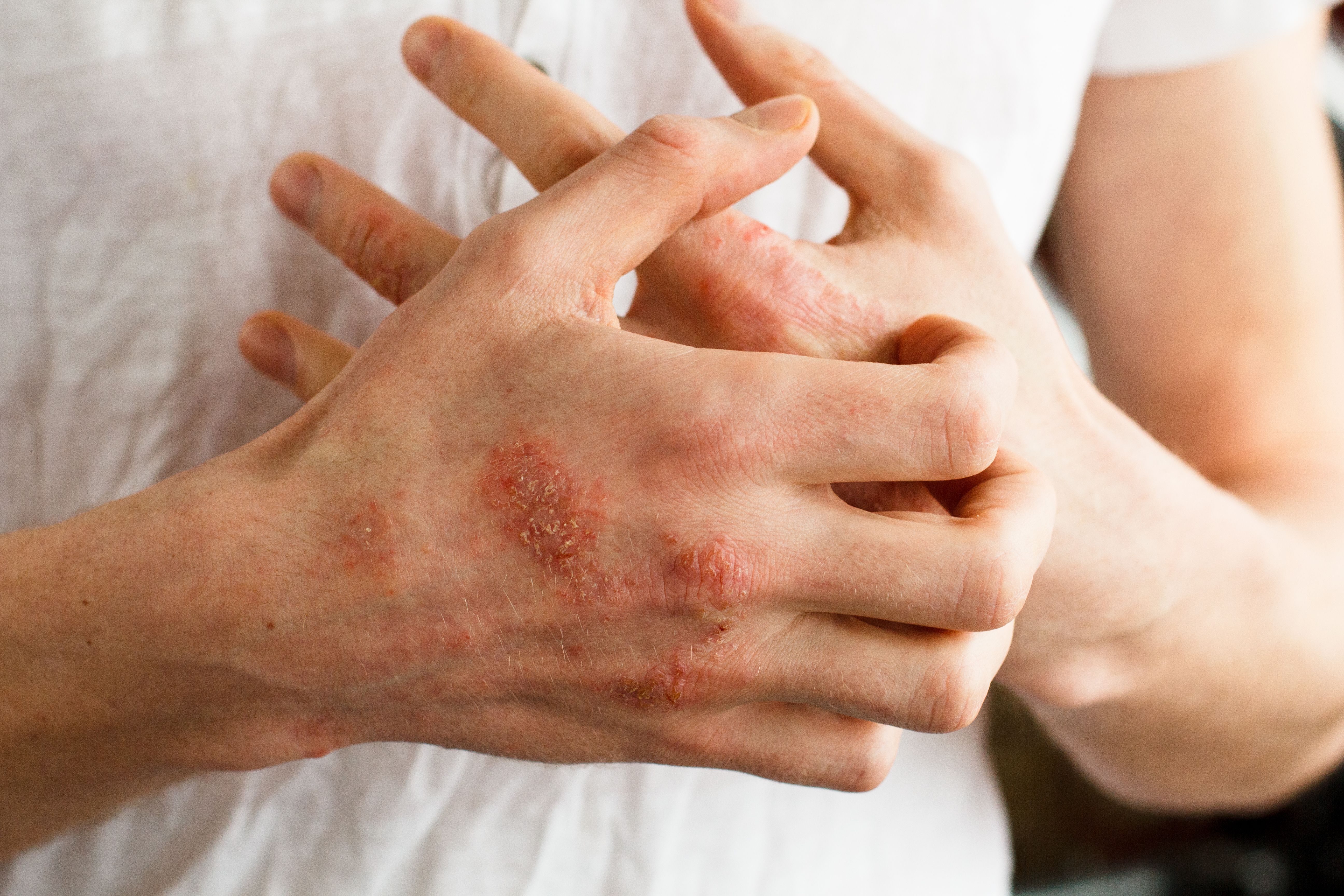
AOBiome Therapeutics, Inc., announced the results via a press release,1 sharing details of the company’s phase 2b trial evaluating the drug. The study2 was published in eClinicalMedicine by The Lancet.
Within the randomized, double-blind, placebo-controlled, dose-ranging trial, 547 adult patients ages 18 to 65 were included for participation. Prospective participants were required to have mild-to-moderate AD and moderate-to-severe pruritus in order tobe enrolled in the study. Across 56 study sites in the US, participants were randomized using a 1:1:1 ratio to either a low dosage of B244, a high dosage of B244, or a vehicle.
Participants receiving the low dosage of B244 received an optical density (OD) at 6.00 nm with OD 5.0. Participants receiving the high dosage of B244 received OD 20.0. All participants were instructed to apply the topical spray on a twice-daily basis for a duration of 4 weeks. This was followed up by a 4 week follow-up period.
Throughout the study, researchers tracked improvement and safety using the Worst Itch Numeric Rating Scale (WI-NRS), Eczema Area and Severity Index (EASI), and Investigator Global Assessment (IGA). Researchers sought a primary endpoint of an average change in WI-NRS after 4 weeks of treatment.
At the conclusion of the study, researchers found that itch had reduced by 34%, with a 2.8% itch rating scale reduction in patients being treated with B244 and a 2.1% reduction in patients being treated with the placebo. Patients receiving B244 also achieved a clinically meaningful itch response, with 30.8% experiencing an equal to or greater than 4-point reduction in WI-NRS from baseline, as opposed to 21.8% achieving the same metric in the placebo group.
Furthermore, researchers noted overall improvements in eczema severity, with 29.3% of high-dosage B244 patients and 27.7% of low-dosage B244 patients achieving EASI75 by the conclusion of the 4 weeks. EASI75 was achieved in only 15.8% members of the vehicle-control group.
Additionally, 26.2% of high-dosage B244 patients and 21.7% low-dosage B244 patients achieved an equal to or greater than 2-point improvement in IGA, while this was achieved in only 12.3% of patients receiving the placebo treatment.
Researchers reported the drug to be well-tolerated in participants, without serious adverse effects. Treatment-emergent adverse events were low in incidence, mild in severity, and transient, with headache being the most frequently-occurring.
"B244 is a promising new non-steroidal therapy for AD, with a very novel mechanism of action,” said Jonathan Silverberg, MD, PhD, MPH, in the press release.1 Silverberg is director of clinical research at the George Washington University School of Medicine and Health Sciences. “The study was well designed and the results are very promising. B244 may address a number of unmet needs for atopic dermatitis patients."
References
- BioSpace. AOBIOME announces the publication of positive phase 2B results in the Lancet’s eclinicalmedicine for B244 in the treatment of mild-to-moderate atopic dermatitis and moderate-to-severe pruritus. BioSpace. May 16, 2023. Accessed May 16, 2023. https://www.biospace.com/article/releases/aobiome-announces-the-publication-of-positive-phase-2b-results-in-the-lancet-s-eclinicalmedicine-for-b244-in-the-treatment-of-mild-to-moderate-atopic-dermatitis-and-moderate-to-severe-pruritus/.
- Silverberg J, Lio P, Simpson E, Li C, et al. Efficacy and safety of topically applied therapeutic ammonia oxidising bacteria in adults with mild-to-moderate atopic dermatitis and moderate-to-severe pruritus: a randomised, double-blind, placebo-controlled, dose-ranging, phase 2b trial. eClinMed. Published May 16, 2023. Accessed May 16, 2023. https://doi.org/10.1016/j.eclinm.2023.102002
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











