- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
Real-World Cohort Study to Compare Efficacy and Safety of Adalimumab Biosimilars for Psoriasis
Author(s):
Study results are expected to be presented at international dermatology conferences by the end of the year.
The largest real-world cohort study1 evaluating the efficacy and safety of adalimumab biosimilars is currently underway.
pimentos/AdobeStock
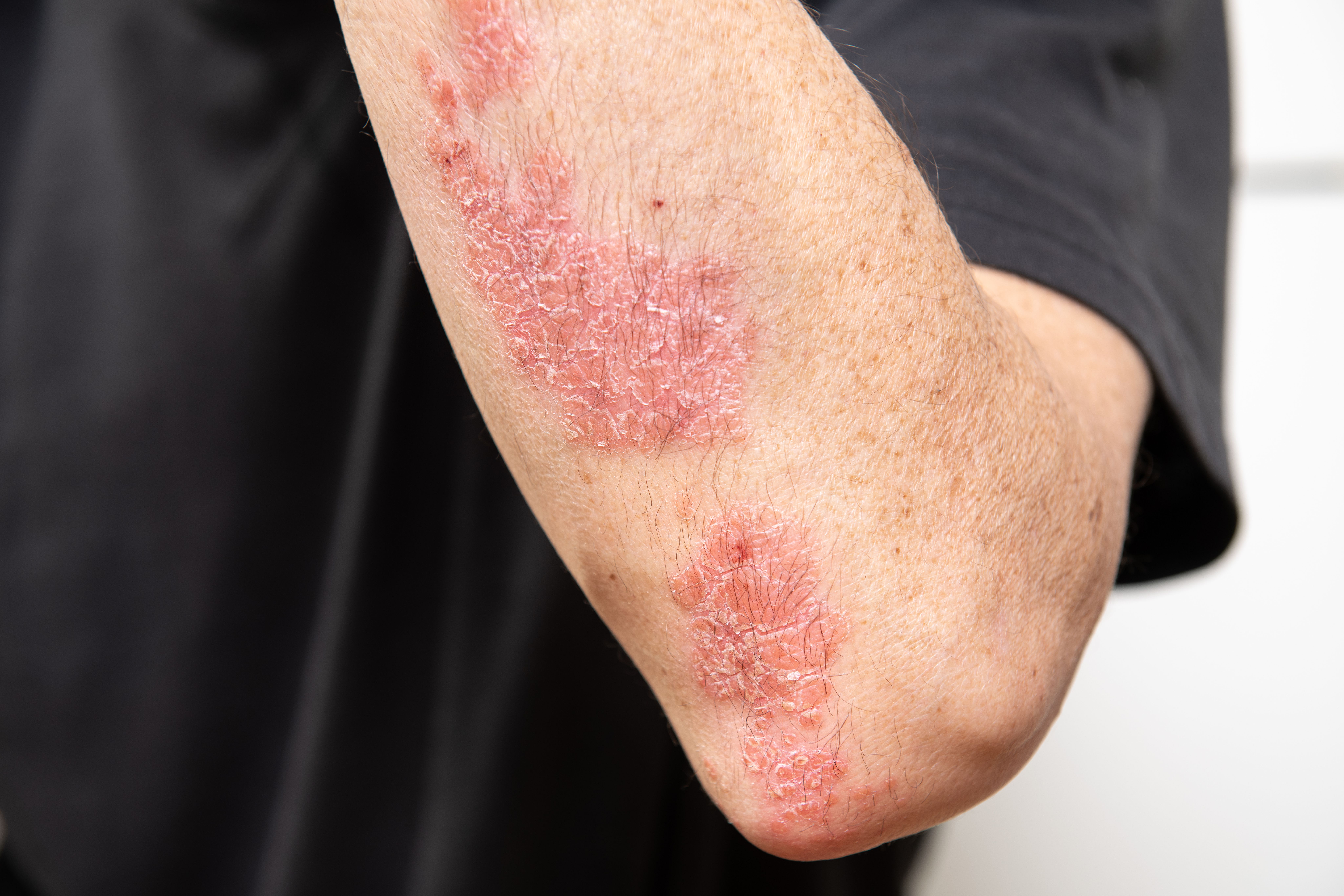
The multinational federated cohort study began in May 2023 and is expected to conclude in December 2023 with results anticipated to be presented at international dermatology conferences by the end of the year. Researchers aim to compare the safety and drug survival of adalimumab biosimilars in the treatment of psoriasis, particularly in routine healthcare settings. According to Phan et al, researchers anticipate there will be no statistical significance or difference reported between biosimilars and originator adalimumab with regard to both safety and drug survival.
“This evaluation can directly inform clinical decision-making and help identify the optimal treatment options for psoriasis. Furthermore, assessing the risk of adverse events associated with adalimumab biosimilars in real world settings is crucial to inform patients and healthcare providers, as clinical trials may not have adequately estimated this risk,” according to Phan et al. “The results of such evaluations can either reassure patients and clinicians of the effectiveness and safety of biosimilars and enhance confidence in their use or help identify any differences between currently available adalimumab products.”
Prior to the study, researchers conducted an online survey to assess the concerns associated with use of biosimilars. Results showed that 89% of respondents were significantly concerned about using adalimumab biosimilars for psoriasis treatment, with 68% expressing concerns for biosimilar safety and 78% expressing concern for biosimilar efficacy.
Researchers then conducted a small focus group with 5 patients being treated with biosimilars for psoriasis.
Study participants will include patients with plaque-type psoriasis of all ages and sexes. Patients will need to either be actively treated with adalimumab originator Humira or with a biosimilar.
“This study is anticipated to be the largest real-world cohort study investigating the effectiveness and safety of adalimumab biosimilars for the treatment of psoriasis. Using both routinely collected healthcare data and data from dedicated pharmacovigilance registries within the PsoNet initiative, this study draws on a diverse patient population from multiple countries, ensuring robust generalisability of study findings,” study authors wrote. “To minimise selection bias and maximise the accuracy of study results, this investigation employs a prevalent new-user cohort design, which captures both switchers from adalimumab originator to biosimilars and patients who initiated treatment directly with adalimumab biosimilars. By accounting for previous adalimumab exposure, the study design controls for potential selection bias, confounders and enhances the validity of the observed outcomes. Overall, the findings of this study will provide valuable insight into the real-world effectiveness and safety of adalimumab biosimilars for psoriasis treatment, enabling informed clinical decision making for patients and healthcare providers.”
Reference
- Phan DB, Jourdain H, González-Quesada A, et al. rug survival and safety of biosimilars and originator adalimumab in the treatment of psoriasis: a multinational cohort study. BMJ Open. 2023;13:e075197. doi: 10.1136/bmjopen-2023-075197





