- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
RelabotulinumtoxinA Significantly Improved Glabellar and Lateral Canthal Lines in READY-3 Trial
Author(s):
This data builds upon data of the READY-1 and READY-2 trials, wherein relabotulinumtoxinA's duration of effect was validated.
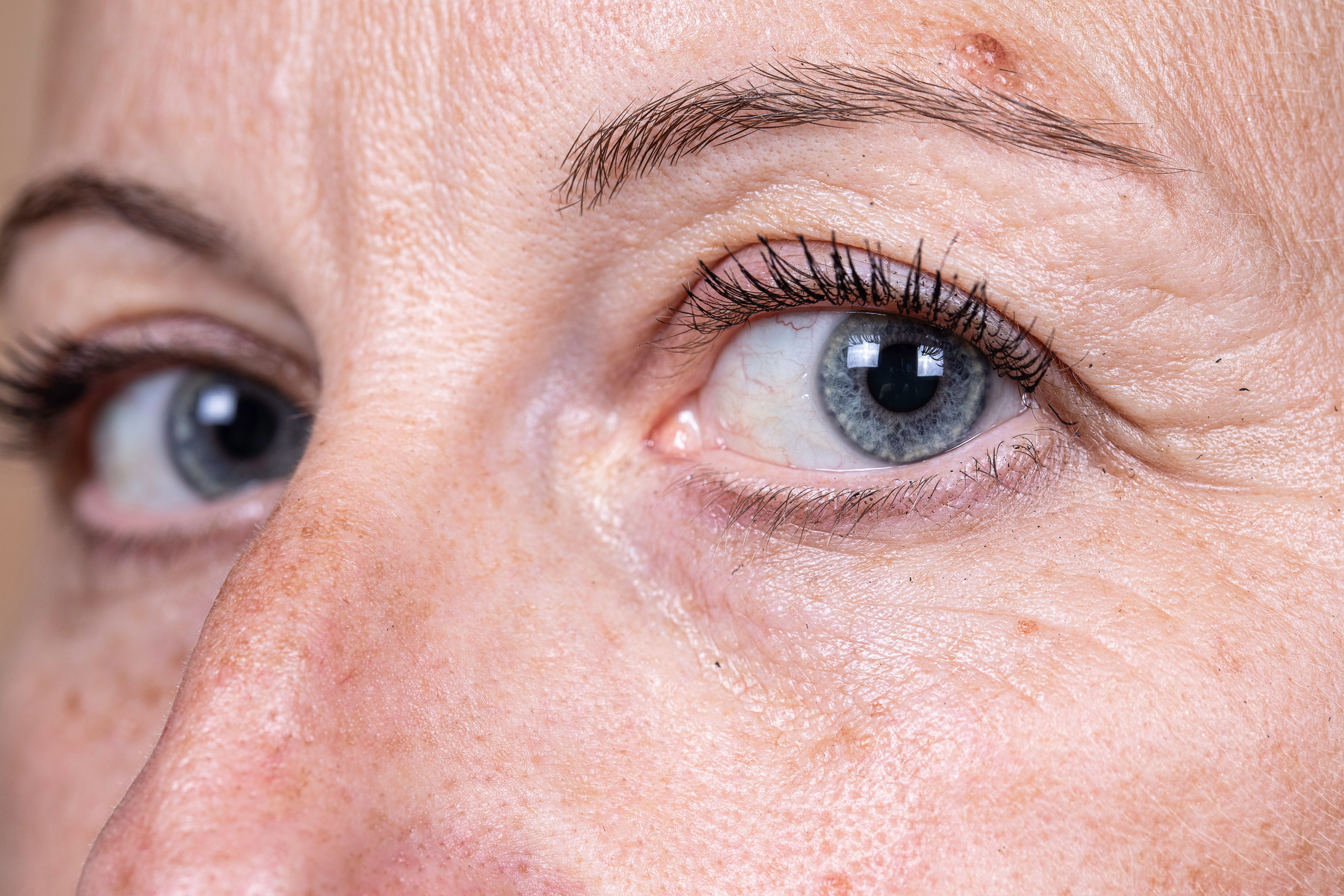
Galderma recently announced positive topline results from its phase III READY-3 clinical trial. The study focused on the use of relabotulinumtoxinA, a promising treatment for glabellar lines (frown lines) and lateral canthal lines (crow's feet).
According to Galderma, relabotulinumtoxinA demonstrated efficacy when administered alone or simultaneously for both frown lines and crow's feet.
The READY-3 study, a randomized, double-blind, placebo-controlled trial, enrolled over 1,900 participants. The co-primary endpoints were met, with relabotulinumtoxinA significantly improving both frown lines and crow's feet. The results were particularly promising, with a lasting duration of effect observed for 6 months.
Patients and investigators reported at least a 2-grade improvement in line severity after treatment, with high response rates at one month. Investigator-reported response rates were impressive, with 94% to 96% reporting none or mild frown lines and 79% to 84% reporting none or mild crow's feet when treated alone or simultaneously.
The safety profile of relabotulinumtoxinA was favorable, with low rates of treatment-related adverse events reported. Patient satisfaction was notably high, with up to 91% reporting satisfaction with simultaneous treatment at one month.
The data presented not only solidify the success of the READY-3 trial but also contribute to the overall positive trend seen in the broader READY phase III clinical trial program, according to a press release. Galderma is set to share these findings at the TOXINS 2024 International Conference in Berlin, scheduled from January 17 to 20, 2024.
Baldo Scassellati Sforzolini, MD, PhD, MBA, global head of R&D at Galderma, expressed enthusiasm about the upcoming conference, stating, "We're looking forward to sharing the latest updates from our world-leading injectable aesthetics portfolio at TOXINS 2024, particularly first data from our phase III READY-3 trial. These results build on those previously seen in the READY-1 and READY-2 trials, further reinforcing RelabotulinumtoxinA’s ability to provide long-lasting improvement for frown lines and crow’s feet when treated simultaneously."
The results from READY-3 not only complement but also reinforce the positive findings from the READY-1 and READY-2 trials. These studies, along with recent phase IIIB results, showcased the rapid onset of action of relabotulinumtoxinA on frown lines and crow's feet as early as day 1.
"Patients often want their aesthetic look to last longer or to receive treatment on multiple facial areas simultaneously, and practitioners want to deliver consistent results for their patients," said Vince Bertucci, MD, FRCPC, co-director of Dermatologic Laser Surgery & Aesthetic Dermatology Fellowship at the University of Toronto, Canada. "These new data highlight the ability of RelabotulinumtoxinA to address all these key needs for frown lines and crow’s feet, while also being a ready-to-use formulation that’s easy to administer."
Reference
Galderma. TOXINS 2024: Galderma’s phase III relabotulinumtoxinA results demonstrate positive efficacy and long duration of effect when treating frown lines and crow’s feet simultaneously. BioSpace. January 17, 2024. Accessed January 17, 2024. https://www.biospace.com/article/releases/toxins-2024-galderma-s-phase-iii-relabotulinumtoxina-results-demonstrate-positive-efficacy-and-long-duration-of-effect-when-treating-frown-lines-and-crow-s-feet-simultaneously/#:~:text=The%20study%20met%20its%20co,of%20effect%20for%20six%20months
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.