- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Revance injectable outperforms Botox
According to early research, not-yet-approved RT002 (DaxibotulinumtoxinA for Injection, Revance Therapeutics) lasts longer, might be safer and offers more dramatic wrinkle reduction than today’s most popular neurotoxin Botox (Allergan).
According to early research, not-yet-approved RT002 (DaxibotulinumtoxinA for Injection, Revance Therapeutics) lasts longer, might be safer and offers more dramatic wrinkle reduction than todayâs most popular neurotoxin Botox (Allergan).
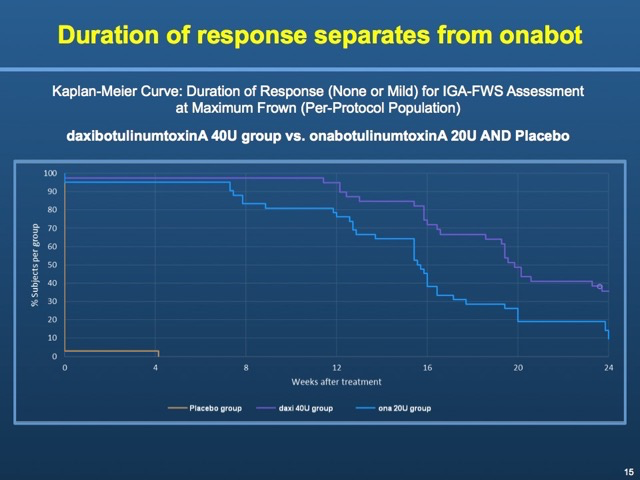
Phase 2 clinical results, presented in March 2016 at the American Academy of Dermatology (AAD) annual meeting, show that three dose levels of RT002 resulted in at least 1-point improvement in frown lines based on the Investigator Global Assessment-Facial Wrinkle Severity (IGA-FWS) scale at four weeks. Revance's study also showed a six-month median duration of effect for RT002.
The effect from the mid-dose level of RT002 Injectable 40U lasted 23.6 weeks, compared to 20U of Botox lasting 18.8 weeks. An investigator assessment of none to mild wrinkles suggests RT002 Injectable 40U was superior in most cases to Botox. At six months, for example, 31 percent of subjects in the Revance group maintained no wrinkles to mild wrinkles, compared to 12 percent in the Botox arm, according to a company press release about the BELMONT phase 2 active comparator study.
Separately, independent research conducted in 2001 by Jean Carruthers, M.D., clinical professor of ophthalmology, University of British Columbia, and botulinum toxin pioneer, on Botox had shown that increased dosing does not result in meaningfully longer duration.
Safety-wise, RT002 appeared generally safe and was well-tolerated. Adverse events were predominantly localized, transient and mild, with no serious adverse events or evidence of any systemic exposure at any of the three doses evaluated, according to the release.
Dr. Carruthers and her husband Alastair Carruthers, M.D., performed both female and male dose ranging studies with onabotulinumtoxinA published in 2001. This is the female dose ranging study which shows that if you simply up the dose of onabotulinumtoxinA in the glabella you do not get increased longevity of response, according to Dr. Jean Carruthers. Photo: Jean Carruthers, M.D.
Revance intends to bring the RT002 40U dose to phase 3 clinical studies, expected to start after June 2016.
NEXT: Notable differences
Notable differences
Some differences between RT002 and other neurotoxins: RT002 combines Revance's proprietary botulinum toxin type A molecule, which is free of other proteins or animal-derived components, with the companyâs patented TransMTS peptide technology. The combination offers targeted delivery to intended treatment areas, while reducing potential spread beyond the injecting site, according to Revance.

Dr. CarruthersDr. Carruthers was principal investigator and presented the BELMONT phase 2 study findings at AAD. Researchers enrolled 268 subjects with moderate to severe glabellar lines and looked at three RT002 doses, 20U, 40U or 60U.
âDaxibotulinumtoxinA (RT002) is an intriguing neuromodulator because of its unique structure and its considerably longer effect. Because it has a unique âfurry overcoat,â namely, the TransMTS peptide technology, it seems to stay anchored in the location it was injected. It is believed this âanchoringâ generates the longer duration,â Dr. Carruthers tells Dermatology Times.
In the BELMONT study, according to Dr. Carruthers, there was no ptosis in the 20U and 40U groups, compared to a 1.9% incidence in the Botox 20U group and 7.5% incidence in the 60U daxibotulinumtoxinA group. There was no male ptosis with the 60U group, which may indicate that the male glabella can handle more units.
âThis, in itself, is a welcome safety aspect,â she says.
In addition, at week 24 of the study, 35.9% of the daxibotulinumtoxinA 40U group still had 1-point improvement on the global scale compared to 19% of the onabotulinumtoxinA group.
âAs we physicians have all known and respected onabotulinumtoxinA for its excellent effect on our patients for many years, it is a welcome and wonderful surprise to find another neuromodulator which exceeds our expectations of both safety and longevity of response,â Dr. Carruthers says.
If approved, it could be three to four years before RT002âs North American phase 3 trial and the FDAâs and Health Canadaâs considerations of those results, according to Dr. Carruthers.
Whether the new neurotoxin on the block will replace those on the market remains to be seen.
âIn our experience, patients become very loyal to the neuromodulator that has been giving them such excellent results over many years. I donât see daxibotulinum replacing currently approved neuromodulators, but rather expanding the aesthetic market, particularly for those patients looking for longer-lasting effects,â she says. âI think dermatologists will need to understand the mechanisms by which this new neuromodulator is able to provide enhanced safety and longevity for their patients. However, it should not be a steep learning curve.â

Dr. ZeichnerJoshua Zeichner, M.D., director of cosmetic and clinical research and assistant professor of dermatology at The Mount Sinai Hospital, New York, N.Y., says that while there are several different toxins currently available--each with slightly different characteristics including speed of onset, diffusion and duration of activity--a new toxin that offers patients a longer lasting effect would be a welcome addition.
â[RT002] demonstrated a longer duration of activity compared to currently available toxins, which in the real world would offer patients more convenience, with fewer visits to the office and fewer needle sticks,â Dr. Zeichner says.
Additional information about the trial can be found at www.clinicaltrials.gov, Clinical trial identifier NCT02303002.
Disclosures: Dr. Carruthers does research for and consults with Revance, Allergan, Merz, Alphaeon and Zeltiq. Dr. Zeichner reports no relevant disclosures.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.














