- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Roflumilast Foam, 0.3% for Seborrheic Dermatitis Launches in US
Author(s):
Zoryve was approved for the treatment of seborrheic dermatitis in patients ages 9 years and older in December.
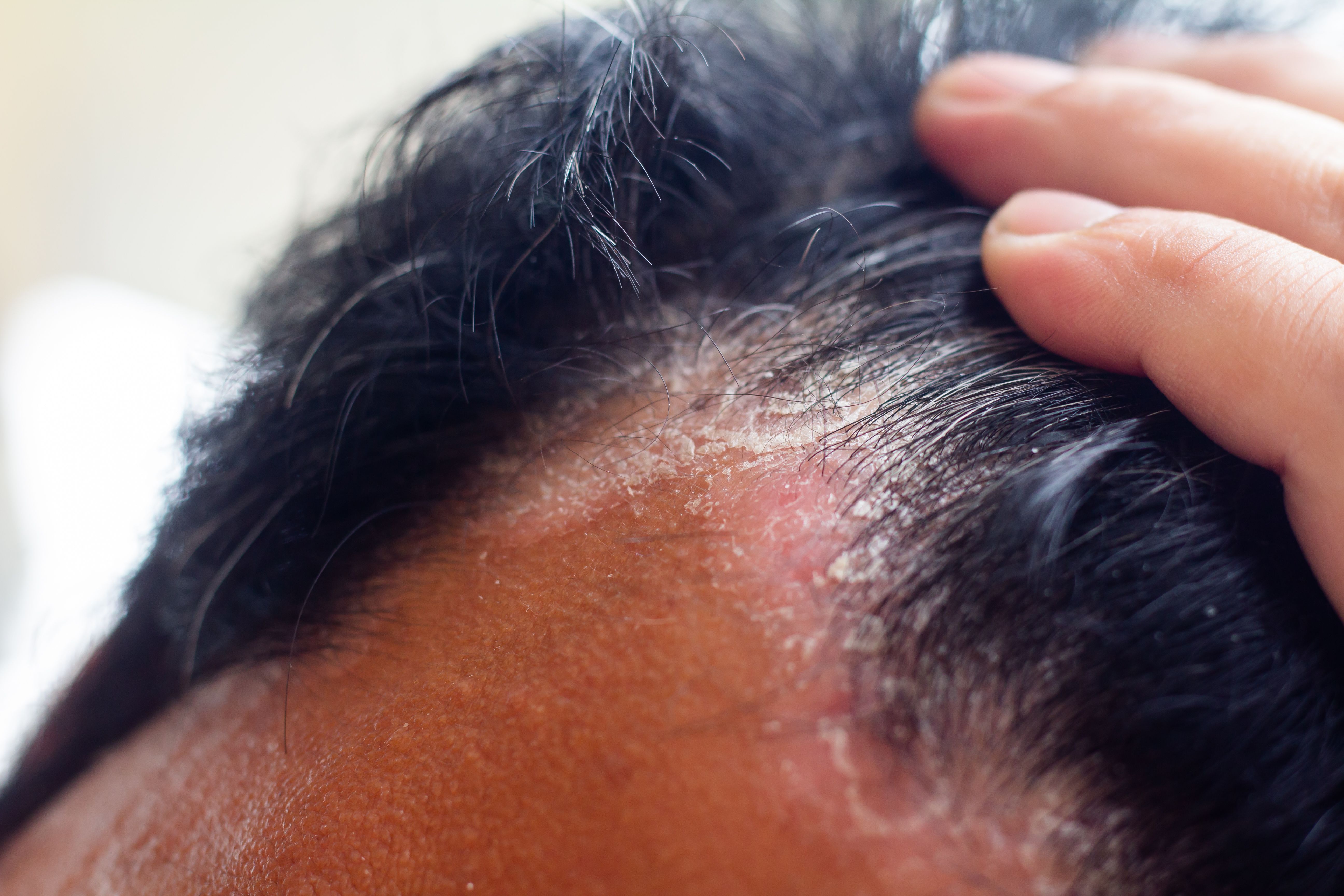
Arcutis Biotherapeutics announced today1 the US launch of roflumilast (Zoryve) topical foam, 0.3% for the treatment of seborrheic dermatitis in adults and children 9 years of age and older.
Roflumilast topical foam was approved by the US Food and Drug Administration for this indication in December,2 making the once-daily steroid-free foam the first drug approved for seborrheic dermatitis in more than 2 decades. In the roflumilast foam-treated group of the pivotal phase 3 STRATUM study (NCT04973228), almost 80% of patients successfully met the primary efficacy endpoint of IGA Success by week 8, with slightly over 50% achieving complete clearance.
Read more from Dermatology Times about the approval here.
Roflumilast topical foam will be available via pharmacy and wholesale channels this week. Arcutis will hold a conference call today, Monday, January 22, at 1:30 PM PST.
“Following decades without significant innovation in seborrheic dermatitis treatment, it’s exciting to have an approved, targeted treatment option for such a common yet burdensome inflammatory disease. ZORYVE foam possesses several unique qualities that address unmet needs of seborrheic dermatitis patients. Notably, ZORYVE foam offers once-daily application, a water-based foam vehicle that can be used anywhere on the body, and versatility for use across all skin and hair types as well as the full spectrum of disease severity,” said Raj Chovatiya, MD, PhD, MSCI, a dermatologist, clinical investigator based in Chicago, and Dermatology Times Editorial Advisory Board member, in a press release.1
“In clinical trials, ZORYVE foam provided complete clearance for more than half of all subjects, and 3 in 4 patients achieved IGA treatment success at 8 weeks, with greater than 40% achieving IGA treatment success as early as two weeks," Chovatiya said. "Based on these key attributes, ZORYVE foam has the potential to define a new standard of care for seborrheic dermatitis.”
References
- Arcutis. ZORYVE (roflumilast) topical foam, 0.3%, for the treatment of seborrheic dermatitis launches in the United States. Globe Newswire. Published January 22, 2024. Accessed January 22, 2024. https://www.globenewswire.com/news-release/2024/01/22/2813014/0/en/ZORYVE-roflumilast-Topical-Foam-0-3-for-the-Treatment-of-Seborrheic-Dermatitis-Launches-in-the-United-States.html
- Arcutis. FDA approves Arcutis’ ZORYVE (roflumilast) topical foam, 0.3% for the treatment of seborrheic dermatitis in Individuals aged 9 years and older. Published December 15, 2023. Accessed January 22, 2024. https://www.arcutis.com/fda-approves-arcutis-zoryve-roflumilast-topical-foam-0-3-for-the-treatment-of-seborrheic-dermatitis-in-individuals-aged-9-years-and-older/
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.