- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
SFA-002 Phase 1b Trial Shows Positive Results for Treatment of Psoriasis
Author(s):
In the cohort trial, 85% of patients showed an improvement of more than 50 on PASI.
SFA Therapeutics recently announced positive results from the first of 2 cohorts in their SFA-002 phase 1b clinical trial for treatment of mild to moderate psoriasis.1 The oral pill simultaneously acts on multiple pathways, down-regulating levels of pro-inflammatory cytokines that are involved in the pathogenesis of psoriasis, including tumor necrosis factor-α (TNF-α), interleukin-12, interleukin-23, interleukin-17, and interferon-y. SFA-002 also down-regulates autoimmunity.
hriana/AdobeStock
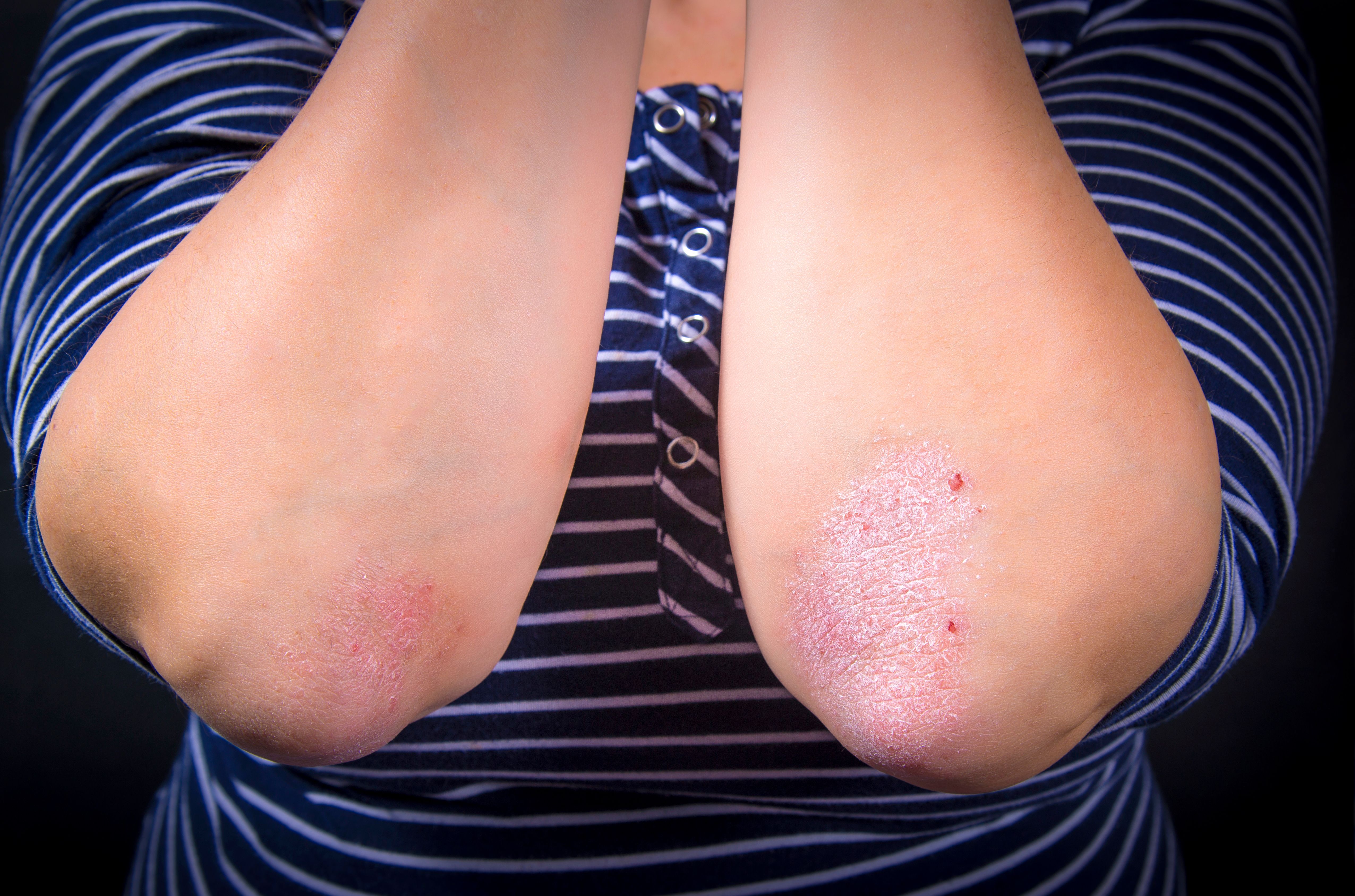
The open-label, prospective study continues to evaluate the safety and efficacy of SFA-002 at 2 dosages in 2 cohorts. The study includes 12 weeks of treatment, 1 month of follow-up, and an optional 12 weeks of extension.
In the cohort 1 analysis of 14 patients, including 6 on the 12-week extension, 85% of patients showed an improvement of greater than 50 on the Psoriasis Area and Severity Index (PASI) score, and 71% of patients demonstrated an improvement of greater than 75 on PASI score at the end of the 1-month follow-up. Two patients achieved PASI 100. Effectiveness of SFA-002 was seen as early as 6 weeks after treatment initiation.
Ira Spector, chief executive officer of SFA Therapeutics, shared results with Dermatology Times® ahead of publication, explaining that the 12-week extension ended August 27, 2023, and results showed that 92% of patients experienced improvement greater than 50 on PASI score.
Patients with scalp psoriasis and palmoplantar psoriasis were included in the study. No adverse events or toxicities occurred in the cohort 1 analysis during treatment or follow-up.
“The outcomes of this research consistently demonstrate remarkable response rates. Introducing a novel oral agent that presents no safety concerns and leads to exceptional responses in psoriasis patients signifies a significant advancement in our therapeutic arsenal,” Daniel N. Sauder, MD, FRCPC, FACP, past chairman of the Department of Dermatology at the Johns Hopkins School of Medicine and past chief of dermatology at the University of Toronto, Ontario, told Dermatology Times.
The second cohort of the phase 1b clinical trial is ongoing (NCT05642182) with 15 additional patients and is expected to be complete by the end of 2023. When the trial is complete, the safety and efficacy of the 2 formulations will be compared and 1 will be selected for phase 2.
Nearly 90% of patients with autoimmune diseases worldwide do not take immunosuppressants due to cost and accessibility, and investigators with SFA Therapeutics are working on solutions, like SFA-002, to ensure accessible treatments are available.
Reference
- SFA Therapeutics announces results for SFA-002 from phase 1b cohort 1 clinical trial for treatment of mild-to-moderate psoriasis. News release. August 14, 2023. Accessed August 30, 2023. https://www.globenewswire.com/en/news-release/2023/08/14/2724489/0/en/SFA-Therapeutics-announces-results-for-SFA-002-from-Phase-1b-Cohort-1-clinical-trial-for-treatment-of-mild-to-moderate-psoriasis.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.







