- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Spesolimab Effective in Treating Generalized Pustular Psoriasis Flares
Author(s):
Boehringer Ingelheim announced that novel antibody treatment can interfere in the IL-36 signaling pathway, reducing flares.
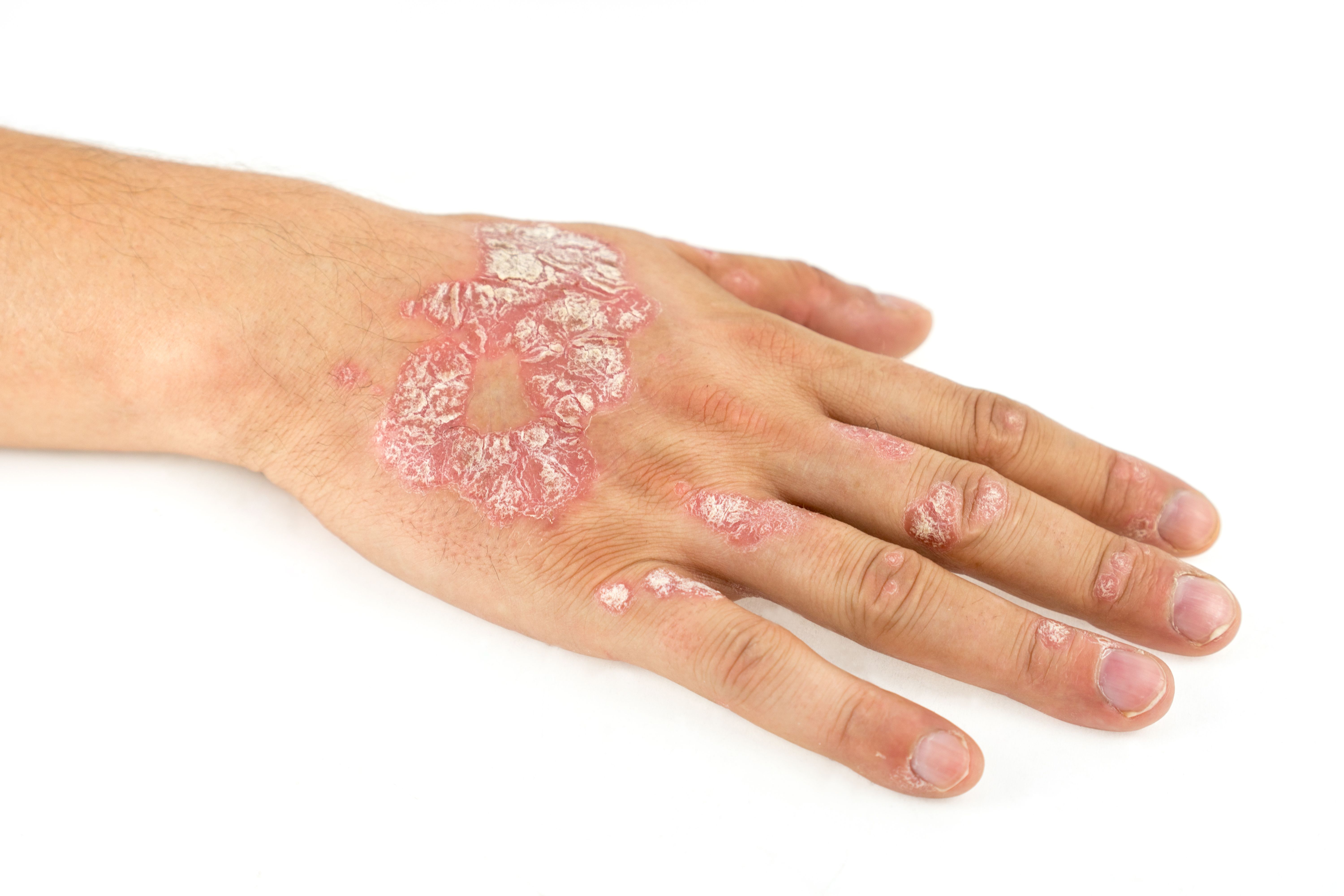
Spesolimab reduced generalized pustular psoriasis (GPP) flares by 84%, Boehringer Ingelheim recently announced. In their Effisayil 2 trial of 123 patients, over the 48-week trial, spesolimab significantly decreased the number of GPP flares compared to placebo.1
In addition, those in the high dose group (n=30) taking spesolimab experienced no flares after 4 weeks of treatment. Spesolimab works by blocking the activation of the interleukin-36 receptor (IL-36R), which is a signaling pathway within the immune system that is part of the pathogenesis of several autoinflammatory diseases, including GPP.
The Effisayil clinical trial program consists of 3 parts. Effisayil 1 was a Phase II study that showed that a single intravenous dose of spesolimab greatly improved symptoms of those experiencing a GPP flare. The results of this trial supported the approval of spesolimab (Spevigo) as the first specific treatment for adults experiencing a GPP flare in major markets. Spesolimab has been approved for treatment of GPP flares in adults in almost 40 countries. It recently was given Breakthrough Therapy Designation (BTD) by the FDA.
Effisayil 2 was a Phase IIb study that has shown a reduction of 84% in GPP flares by those in the high dose group.
Effisayil ON is an open-label extension study to examine the long-term safety and efficacy of spesolimab in those who completed the previous 2 studies.
The Effisayil clinical trial program has examined the largest and broadest population of patients with GPP, specifically evaluating therapy targeting the IL-36 signaling pathway.
“Effisayil 2 is the first and largest multinational randomized clinical trial to evaluate a treatment for the prevention of GPP flares,” said Bruce Strober, MD, PhD, and clinical professor, Dermatology, Yale University and Central Connecticut Dermatology in a press release. “These results provide further compelling clinical evidence for the role IL-36 signaling plays in the pathogenesis of GPP. Moving forward, our hope is that dermatologists not only have a specific treatment for GPP flares, but that we can effectively prevent them in the future.”
Adverse events in the treatment arm of those participants using spesolimab were similar to those given a placebo. Spesolimab is currently being studied for use in other skin diseases caused by the IL-36 pathway.
Reference
- Spesolimab prevented generalized pustular psoriasis flares in Effisayil 2 trial. News Release. Boehringer Ingelheim. July 4, 2023. Accessed July 10, 2023. https://www.boehringer-ingelheim.com/us/press-releases/effisayil-2-trial-results
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.