- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
TMB-001 for Congenital Ichthyosis Subtypes Tested by Dermatologists
Author(s):
Yale School of Medicine is a testing site for a potential new congenital ichthyosis treatment.
Timber Pharmaceuticals' TMB-001, a topical isotretinoin, received breakthrough therapy designation from the US Food and Drug Administration (FDA) in Q2 2022 for the treatment of congenital ichthyosis (CI) subtypes. As 3 clinical trials have now been conducted across the US to evaluate TMB-001, Yale School of Medicine has been an integral part of TMB-001's development as the only US site involved in all 3 trials.1
“Yale itself has been very instrumental in the clinical studies,” said Christopher Bunick, MD, PhD, associate professor of dermatology at Yale School of Medicine and principal investigator of the CI trials at Yale. “First, the Visual Index for Ichthyosis Severity (VIIS) scale system being used as one of the primary endpoints was developed at Yale by Leonard Milstone and Keith Choate, both Yale professors. Second, Yale is the only site in the US to be a part of all 3 clinical trials (phase 2a, 2b, and now 3), of which I have been the principal investigator."
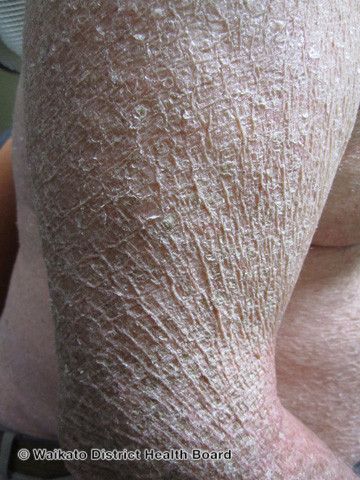
CI commonly presents as dry, thickened, and scaling skin that develops when patients are young. CI can change the way new skin generates by decreasing the shedding of old skin cells or increasing the production of new skin cells.1 Daily life for patients and their caregivers often becomes difficult with extremely dry skin, itching, and sometimes painful fissures across the skin.
“Growing up, I always had to have an extreme regimen of moisturizers in my daily routine, sometimes twice a day in the winter,” said Saiaditya Badeti, an MD and PhD student at Rutgers University New Jersey Medical School and a TMB-001 clinical trial participant. “We would go through huge bottles of Aquaphor, CeraVe, Cetaphil, and Nivea; we would finish them in a week. When I was a child, I used to think that's what everyone did. When I learned that many people just get out of the shower and put clothes on and don't moisturize or put any lotions on, that was really mind-blowing for me.”
The first CI study published in the Journal of the American Academy of Dermatology (JAAD)2 evaluated the safety, tolerability, and efficacy of TMB-001 for the treatment of X-linked or lamellar congenital ichthyosis. The study noted that without current FDA-approved treatments for CI subtypes, systemic retinoids are effective, but can cause dose-limiting adverse events. Participants were treated with 2 different concentrations of TMB-001. Both concentrations were deemed safe, but the lowest concentration had a stronger efficacy signal.
Along with cracked and itchy skin observed with CI, there are significant effects on patient's mental health and body image.
“Physically, patients with ichthyoses generally produce a lot of scale. This scale can cover many parts, if not all parts, of their body and in moderate-to-severe cases, the scale can be thick and constantly shedding. This presents an aesthetic problem, where other people clearly recognize the scale and that the person has a ‘disease’, and this feeds into the emotional challenges. In some patients the scale can be very itchy, or burning/stinging if there are fissures, and areas of the skin may also be very red and irritated in certain patients,” said Bunick. “Emotionally, during childhood there is the stigma of having a disease. Other children won't understand ichthyosis and could bully, make fun of, or minimize the patient. This creates low self-esteem in many patients, or feelings of sadness or inadequacy. Many kids won't be able to participate in similar sports or activities as other kids for fear of ‘being different.’ It could affect the ability to make friends and healthy social circles in school. Adults may experience similar feelings in their job or in trying to establish relationships - having people judge them by their skin, constantly asking what is wrong. There is never a resting moment when someone isn't looking or judging their skin.”
The second CI study also published in JAAD3evaluated the effectiveness of TMB-001 using the VIIS scale and Investigator Global Assessment (IGA) scores. Patients were treated with TMB-001 0.05% or 0.1% compared to vehicle. Bunick noted that 100% of patients who followed protocol achieved a 50% reduction in their VIIS score and a 2-grade improvement in IGA scores with the 0.05% strength.
“I think this is a remarkable achievement and has Timber Pharmaceuticals on the verge of the first FDA-approved medicine for congenital ichthyosis. Moreover, the work by all the Timber trial investigators showed that the topical isotretinoin, even when applied on nearly 90% of the body surface, is safe. We observed little to no lab abnormalities in the blood of the patients, and none that were clinically significant,” said Bunick.

In his personal experience, Badeti had sensitive fissures that would appear on his arms, legs, and neck. Growing up, Badeti struggled with the mental burden CI created as he was self-conscious of how he dressed in public and if his skin was visible. Teenagers used harsh phrases to describe his skin in school as they did not understand the condition. Badeti also spoke on how CI affected his skin differently as a patient with skin of color.
“I've noticed that people with lighter skin tones tend to have more erythema type of variants of ichthyosis. For me, I don't get the erythema as much as the cracking, fissuring, and splitting of the skin,” said Badeti. “I noticed in the initial weeks before starting TMB-001 when they asked me to stop using my normal moisturizers, I developed fissures on my neck. I'm not sure if it was from scratching in the middle of the night, or if it's the skin splitting from having so much buildup of hyperkeratinization and inability to fall off.”
After positive results from the phase 2 trial, Badeti will participate in the phase 3 trials evaluating TMB-001.
“I would encourage other people to also look into Timber’s phase 3 trial if they are eligible and qualified to be included. Ichthyosis as a disease is very rare. Coming across people who are willing to come off of their current regimen that has worked for them and move into something else can be a pretty daunting task, but if they’re willing to take that initiative, I think it helps move studies forward and offer new solutions for other people who may not have had success with their current regimens,” Badeti concluded.
Clinical and Experimental Dermatology has accepted the most recent CI study, which found that TMB-001 was effective in treating both lamellar ichthyosis and X-linked recessive ichthyosis, with all patients meeting VIIS and IGA goals.1
Crucial investigators of Timber Pharmaceuticals’ TMB-001 trials include:
Phase 2a: Neal Bhatia, MD; John Browning, MD, MBA; Christopher G. Bunick, MD, PhD; Amy S. Paller, MD; and Lawrence Charles Parish, MD
Phase 2b: Lindsay Ackerman, MD; Christopher G. Bunick, MD, PhD; Scott Guenthner, MD; Steven Kempers, MD; Kalyani Marathe, MD, MPH; Jeffery Poole, MD; Leslie Castelo-Soccio, MD, PhD; and Joyce M.C. Teng, MD, PhD
Phase 3: Lindsay Ackerman, MD; Christopher G. Bunick, MD, PhD; Joel Lee Cohen, MD; Meghan Couvillion, MD; Kenneth Dawes, MD; Scott Guenthner, MD; Thy Huynh, MD; Joseph Jorizzo, MD; Steven Kempers, MD; Edward Lain, MD, MBA; Lara Wine Lee, MD, PhD, Amy S. Paller, MD; David Pariser, MD; Fernanda Bellodi Schmidt, MD; Patrick Shannon, MD; James Song, MD; Joyce M.C. Teng, MD, PhD; and Albert Yan, MD
References
- Shelton J. Yale tests a treatment for skin diseases that often target children. YaleNews. Published February 9, 2023. Accessed March 9, 2023. https://news.yale.edu/2023/02/09/yale-tests-treatment-skin-diseases-often-target-children
- Paller AS, Browning J, Parish LC, Bunick CG, Rome Z, Bhatia N. Safety, tolerability, and efficacy of a novel topical isotretinoin formulation for the treatment of X-linked or lamellar congenital ichthyosis: Results from a phase 2a proof-of-concept study. J Am Acad Dermatol. 2022;87(5):1189-1191. doi:10.1016/j.jaad.2022.02.060
- Teng JMC, Bunick CG, Guenthner S, et al. The CONTROL study: A randomized, double-blind vehicle-controlled phase 2b study of novel topical isotretinoin formulation demonstrates improvement in recessive X-linked and autosomal recessive lamellar congenital ichthyosis. J Am Acad Dermatol. 2022;87(6):1455-1458. doi:10.1016/j.jaad.2022.07.028
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.