
- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Torii Shares Positive Data From Japan Trial of TO-208 for Molluscum Contagiosum
Author(s):
The confirmatory phase 3 trial demonstrated significant efficacy of TO-208 versus a placebo.
Lukassek/Adobe Stock
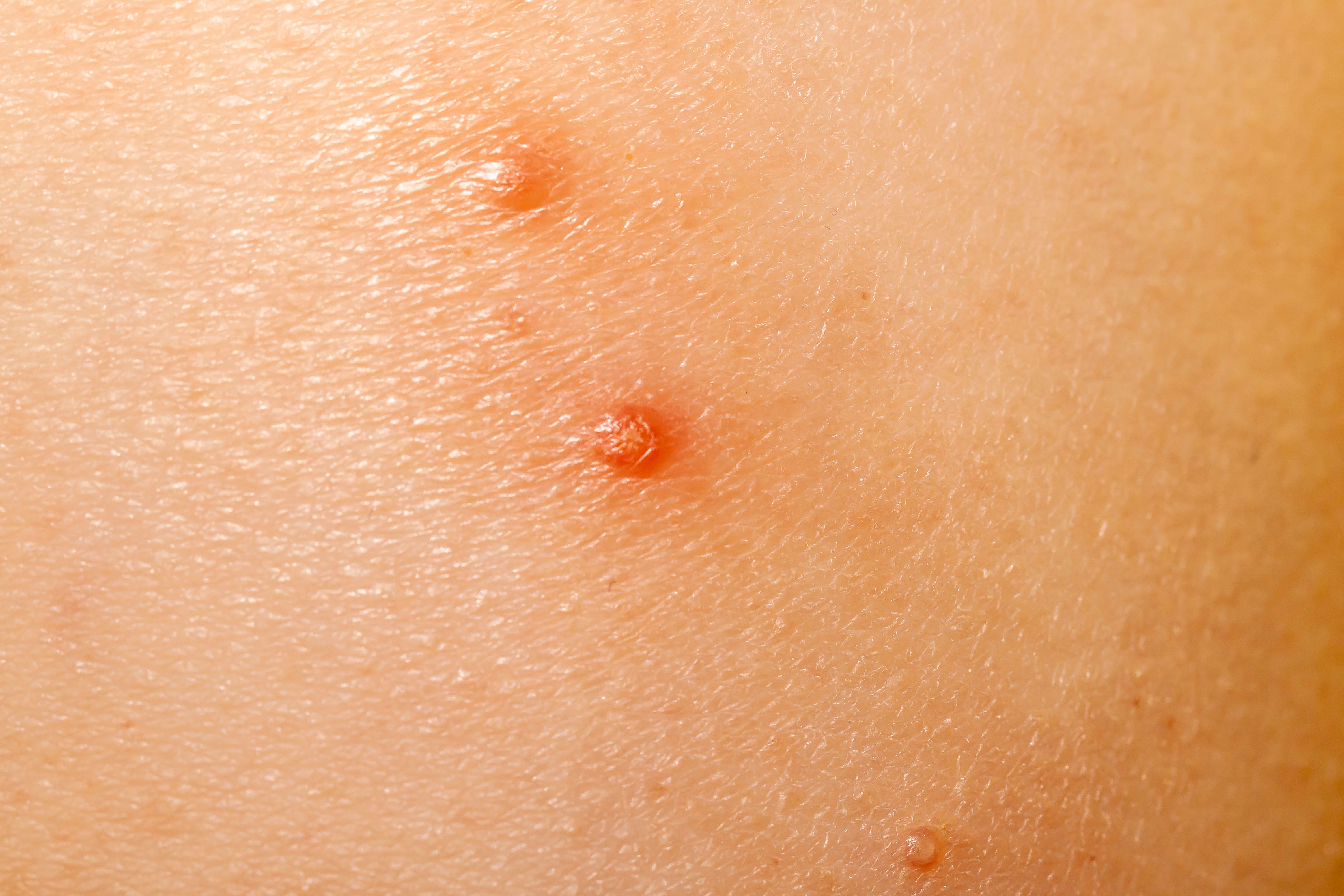
Verrica Pharmaceuticals Inc. and its partner, Torii Pharmaceutical Co., Ltd., today shared positive top-line results from the phase 3 trial of TO-208, also known as VP-102, and marketed as YCANTH in the US, for the treatment of molluscum contagiosum in Japan.
The confirmatory phase 3 trial, conducted in Japan, utilized a double-blind, randomized, parallel-group comparison design to assess the efficacy and safety of TO-208 versus a placebo.
Administered once every 21 days for up to 4 applications, the study aimed to evaluate the clearance of treatable molluscum lesions.
The results demonstrated a statistically significant proportion of subjects achieving complete clearance, the primary efficacy endpoint, compared to the placebo. Safety signals were consistent with previous research, with TO-208 being well-tolerated among participants.
The positive results pave the way for Torii to submit a manufacturing and marketing application for TO-208 in Japan, following the signing of an exclusive licensing agreement signed in March 2021 between Verrica and Torri.
Ted White, the Chief Executive Officer of Verrica Pharmaceuticals, expressed enthusiasm about the promising outcome.
“We are obviously excited by the positive results from this confirmatory Phase 3 trial for TO-208 for the treatment of molluscum in Japan, which underscores the consistent safety and efficacy of VP-102 and FDA-approved YCANTH,” White said in a press release. “We believe Torii is an ideal partner to bring the product to people with molluscum in Japan, and these positive results take us one step closer towards achieving our goal of addressing this large and underserved patient population.”
Reference
Verrica Pharmaceuticals’ development and commercialization partner, Torii Pharmaceutical Co., Ltd., announces positive top-line results from a confirmatory phase 3 trial of to-208 for the treatment of molluscum contagiosum in Japan. Yahoo! Finance. December 15, 2023. Accessed December 15, 2023. https://finance.yahoo.com/news/verrica-pharmaceuticals-development-commercialization-partner-110000548.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.










2 Commerce Drive
Cranbury, NJ 08512
All rights reserved.




