- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Tracking melanoma to p16
Sixty percent of families in which three or more members have melanoma also have a germline mutation in the p16 sequence, which, when functioning properly, inhibits CDK4 and activates RB1 (retinoblastoma). When the p16 function of human melanocytes is blocked, senescence is delayed.

Dr. Bennett, who is a professor of cell biology at St. George's Medical School, University of London, gave the Presidential Lecture at the recent International Pigment Cell Conference in Reston. Along with colleagues, she has been culturing pigmented lesions and immunostaining proteins to investigate relationships between senescence, immortalization, apoptosis and the progression of melanoma.
"Understanding genetic alterations should suggest new targets for therapy and additional markers for diagnosis and prognosis," Dr. Bennett says.

Over the last several decades progress has been made in investigating the number and nature of genetic changes required to produce cutaneous melanoma. Much of the research has examined the similarities and differences in somatic and germline mutations and the role UV exposure plays in somatic mutations.
This has directed attention to the CDKN2A locus on chromosome 9, which encodes two separate tumor suppressors: ARF and p16, which seems more important for melanoma. Indeed, 60 percent of families in which three or more members have melanoma also have a germline mutation in the p16 sequence, which, when functioning properly, inhibits CDK4 and activates R± (retinoblastoma). When the p16 function of human melanocytes is blocked, senescence is delayed, although there is also a higher tendency toward apoptosis if the melanocytes are separated from keratinocytes. (This may be a clue to why early melanomas and dysplastic nevi are thin.)
Senescence
Now research on senescence is merging with older lines of inquiry.
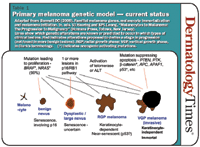
There are two principal types of cell senescence: M1, which is brought on by telomere shortening and is largely p53 dependent, and M0, which is p16-dependent. There is evidence that melanocytes primarily undergo M0 senescence and must overcome this to become malignant.
"As it boils down, if you look at benign nevi, they have all the markers you'd expect of M0 senescence. Nevus cells in culture also appear senescent. So the interpretation is that a mutation has occurred that sets off proliferation in a melanocyte, but this growth becomes halted by senescence, possibly p16-dependent."
Dr. Bennett summarizes her current model
of melanoma progression. First, a BRAF or NRAS mutation causes a melanocyte clone to proliferate and then senesce, forming a benign nevus. It takes one or more mutations in the p16/R± pathway, interfering with M0 senescence, to bring about dysplasia. When M0 is blocked in cultured melanocytes, they grow but then undergo M1-like senescence.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











