- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Tralokinumab Found to be Efficacious in Adolescents With Moderate to Severe Atopic Dermatitis
Author(s):
Results of the phase 3 ECZTRA 6 trial found that the efficacy of the drug was maintained by more than 50% of patients at 52 weeks.
Interleukin-13–targeted treatment with tralokinumab (Adbry; LEO Pharma)was efficacious and well tolerated in adolescents with moderate to severe atopic dermatitis (AD), according to the results of a phase 3 ECZTRA 6 randomized clinical trial.1
Slepitssskaya/AdobeStock
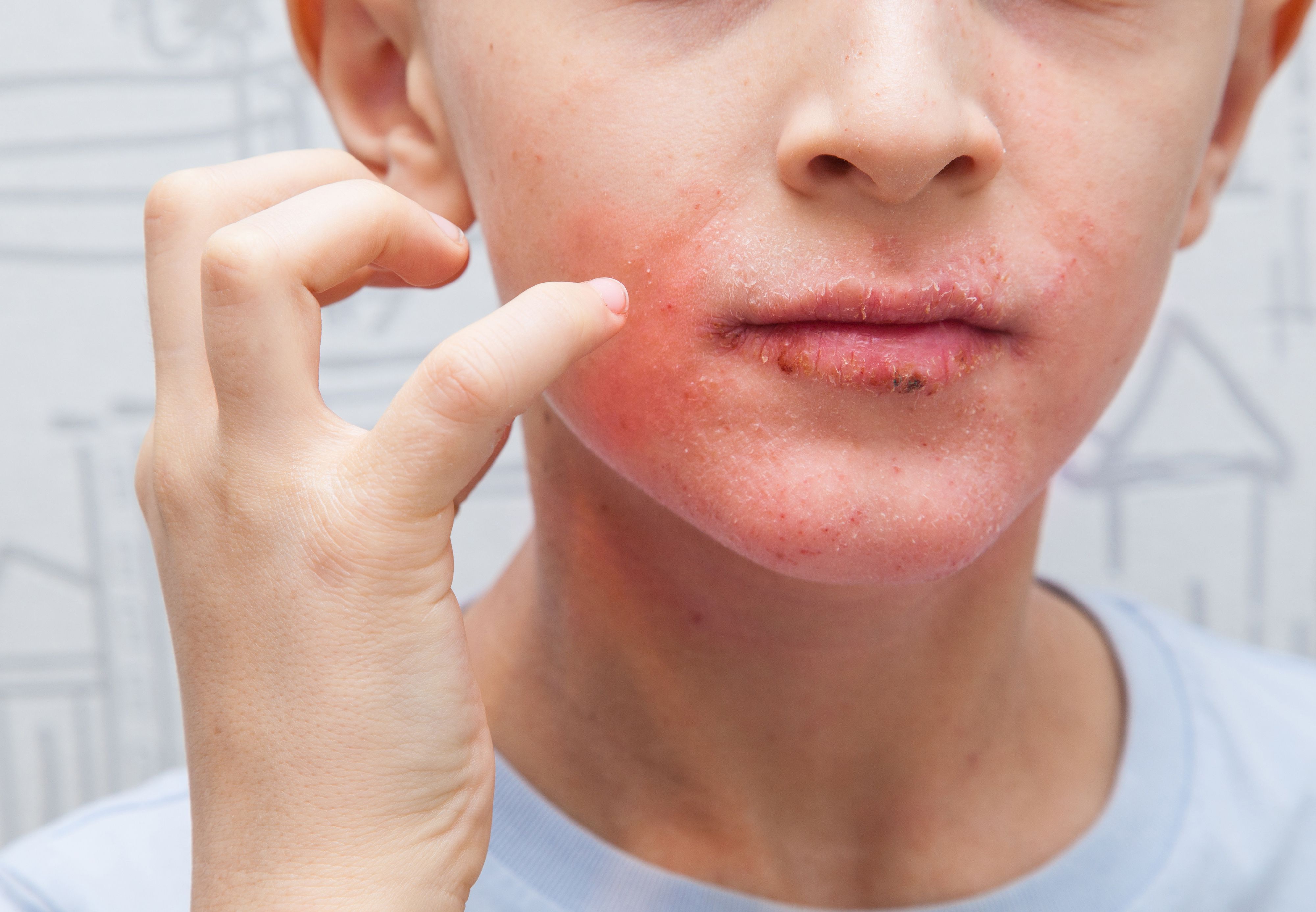
Lead author Amy S. Paller, MD, chair of the department of dermatology and director of the Skin Biology and Diseases Resource-Based Center of the Northwestern University Feinberg School of Medicine and attending physician at the Ann and Robert H Lurie Children's Hospital of Chicago, reported the results of the trial on tralokinumab for pediatric patients with AD who were aged 12 to 17 years.
The 52-week, randomized, double-blinded, placebo-controlled, phase 3 ECZTRA 6 trial was conducted from July 17, 2018, through March 16, 2021, at 72 centers across 10 countries in North America, Europe, Asia, and Australia. Enrolled patients were 12 to 17 years old with moderate to severe AD (Investigator’s Global Assessment [IGA] score ≥3; Eczema Area and Severity Index [EASI] ≥16).
The patients were randomly assigned to tralokinumab (150 or 300 mg) or placebo every 2 weeks for 16 weeks. Patients with an IGA score of 0 (clear) or 1 (almost clear) and/or 75% or higher improvement in EASI (EASI 75) at week 16 without rescue medication received maintenance treatment; other patients switched to open-label tralokinumab, 300 mg, every 2 weeks. Patients receiving placebo who met the primary end point(s) at week 16 without use of rescue medication continued to receive blinded placebo every 2 weeks until week 52.
At 16 weeks, the main goals of the phase 3 trial were for the participants to report an IGA score of 0 or 1 (clear or almost clear) and/or to report an EASI 75 score. The team’s secondary objectives were to report changes in Children's Dermatology Life Quality Index from baseline to week 16, a reduction of 4 or more on the Adolescent Worst Pruritus Numeric Rating Scale, and changes in SCORing AD.
Both tralokinumab doses resulted in improvements in eczema-related sleep NRS vs placebo at week 16. Additionally, Patients receiving tralokinumab, 300 mg, every 2 weeks experienced a greater decrease in Hospital Anxiety and Depression Scale from baseline vs placebo at week 16.
Tralokinumab was well tolerated, with a majority of adverse events (AE) being mild or moderate in severity. In the initial treatment period, proportions of patients with 1 or more AE were similar among those receiving tralokinumab. The most frequent AEs were upper respiratory tract infection, dermatitis atopic (disease exacerbation), injection-site reaction, asthma, and headache.
Study authors said this is believed to be the first study to show that targeting IL-13 alone improved AD signs and symptoms along with improvements in multiple high-effect disease domains in a pediatric population. These results are consistent with tralokinumab data reported in adults with AD and suggest that specific targeting of IL-13 with tralokinumab is an effective and well-tolerated long-term treatment option for uncontrolled AD in adolescents.
Last year, the European Commission extended the marketing authorization for tralokinumab to include adolescents aged 12 to 17 with moderate to severe AD, with US agencies mulling a similar move to broaden the indication.
Reference
1. Paller AS, Flohr C, Cork M, et al. Efficacy and Safety of Tralokinumab in Adolescents With Moderate to Severe Atopic Dermatitis: The Phase 3 ECZTRA 6 Randomized Clinical Trial. JAMA Dermatol. Published online April 19, 2023. doi:10.1001/jamadermatol.2023.0627
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.









