- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Tralokinumab meets endpoints for all phase 3 studies
Author(s):
LEO Pharma has recently announced that all three phase 3 studies examining the safety and efficacy of the atopic dermatitis investigational drug tralokinumab met all of its primary and secondary endpoints, leading way to the company now seeking marketing authorization for the drug.
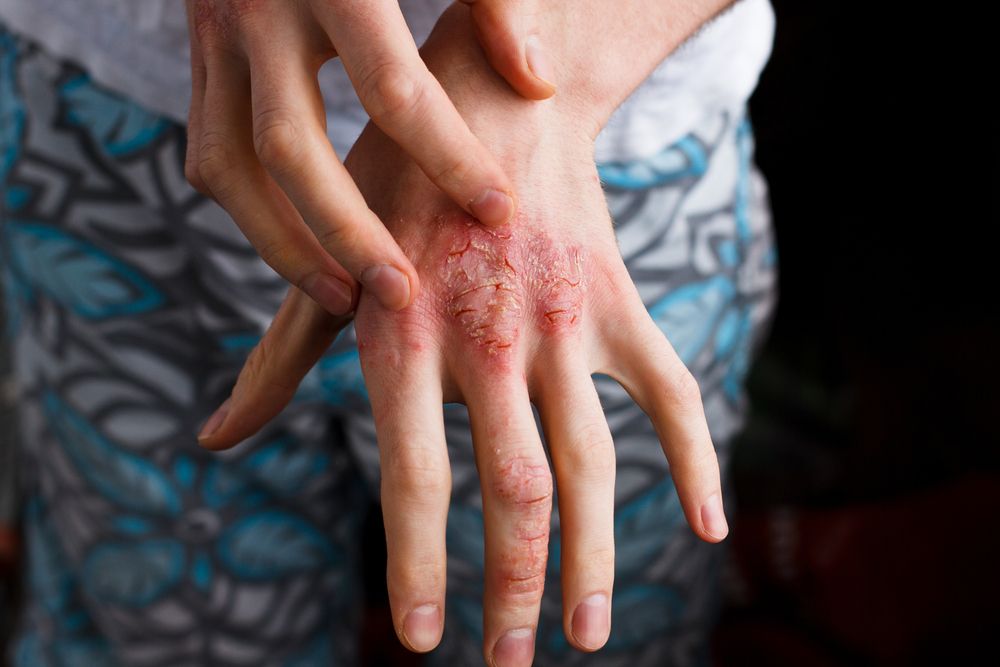
A recent announcement by LEO Pharma reveals their atopic dermatitis (AD) investigational drug, tralokinumab, has taken major steps in multiple phase 3 studies.
RELATED: New ways to tackle itch in atopic dermatitis
Tralokinumab is a monoclonal antibody that aims to offset the interleukin-13 cytokine, one of the believed main contributors of inflammation in AD.
LEO Pharma conducted three separate phase 3 studies (ECZTRA 1, 2 and 3) examining the safety and efficacy of the drug on individuals with moderate-to-severe AD.
Researchers found the three studies met all primary and secondary endpoints. Also, the overall adverse event rate between tralokinumab and the placebo was similar.
“We are encouraged by these study results, which show that tralokinumab could be an efficacious and well-tolerated long-term treatment solution for patients living with this debilitating chronic skin disease,” says Dr. Kim Kjoeller, executive vice president of global research & development, LEO Pharma.
ECZTRA 1 and 2 were double-blind, randomized, 52-week studies that each enrolled nearly 800 adult moderate-to-severe AD patients and examined tralokinumab as a monotherapy. ECZTRA 3 was a double-blind, randomized, 32-week study that observed AD in 380 adult individuals and how the drug acted in conjunction with topical corticosteroids (CTS).
The primary endpoints in all three studies include an Investigator Global Assessment (IGA) score of clear (0) or almost clear (1) at week 16 and a minimum change from the baseline of 75% or greater at week 16. The secondary endpoints include a change from the baseline in week 16 in SCORing of AD (SCORAD), Pruritis Numeric Rating Scale (NRS) of 4 and a Dermatology Life Quality Index (DLQI).
READ MORE: Fast Track designation granted to lebrikizumab for atopic dermatitis
LEO Pharma says their next step will be to apply to regulatory agencies in 2020 for marketing authorization of tralokinumab, as well as release the complete results of the studies at scientific congress presentations and medical journals in the upcoming year.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











