- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
Treatment With Rademikibart Led to Clinically Meaningful Responses in Patients With Atopic Dermatitis
Author(s):
Connect Biopharma announced positive long-term data from its China pivotal trial.
liol88/Adobe Stock
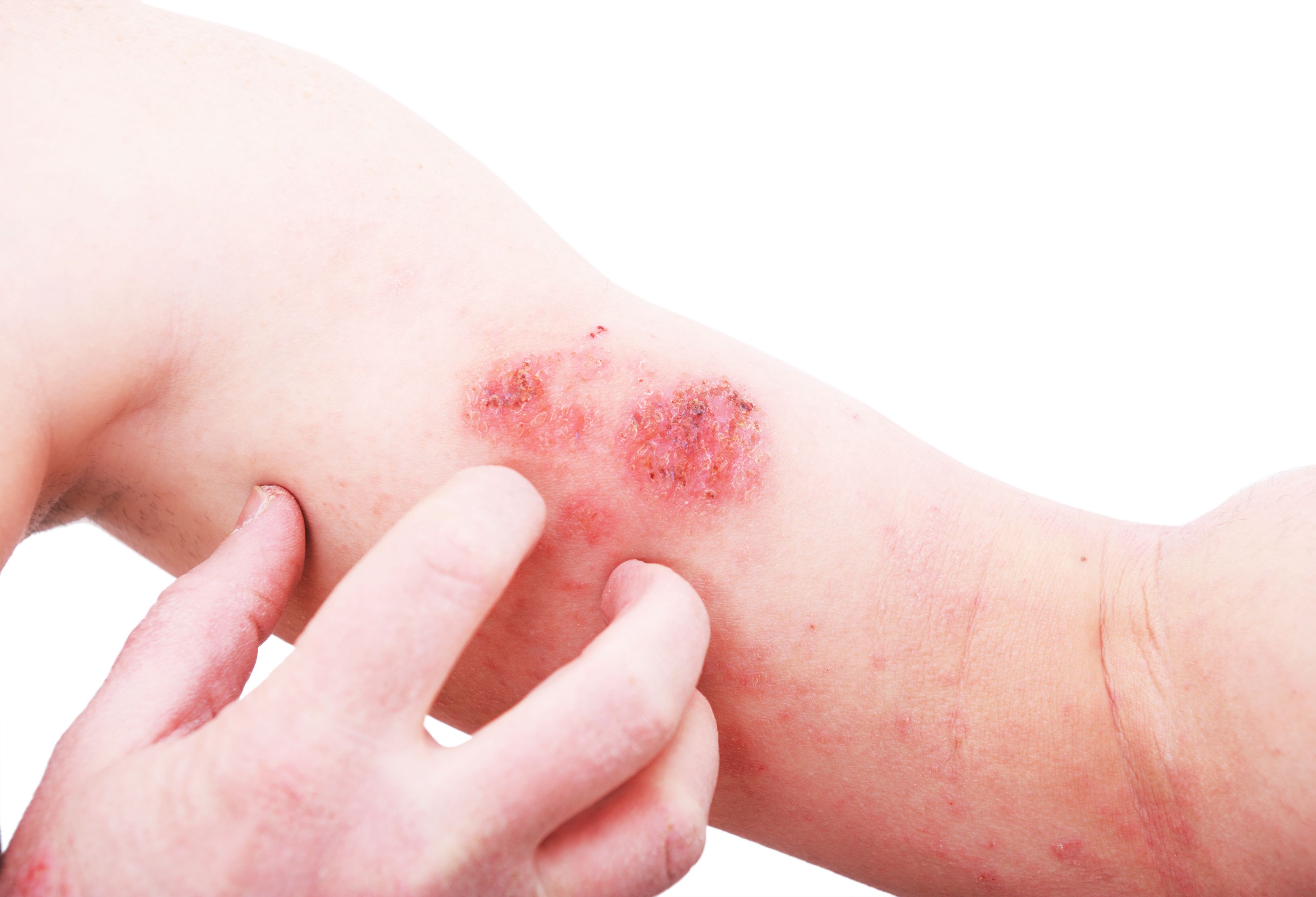
Connect Biopharma announced today positive long-term data from stage 2, or the maintenance phase, of its China pivotal trial of rademikibart in patients with moderate-to-severe atopic dermatitis (AD). Results were consistent with stage 1 findings, wherein rademikibart met all primary and secondary endpoints for this indication.
Patients who had achieved an Eczema Area and Severity Index (EASI)-50 score regardless of initial treatment by week 16 in stage 1 of the trial were randomized to a monotherapy treatment arm of Q2W rademikibart or Q4W rademikibart.
Patients who had not achieved an EASI-50 score by week 16 were assigned to an open-label treatment arm of Q2W rademikibart.
113 patients were treated with Q2W rademikibart, 112 were treated with Q4W rademikibart, and 86 were a part of the open-label treatment arm.
Of patients who had achieved an EASI-75 score or an Investigator's Global Assessment (IGA) score of 0 or 1 at week 16, 76% to 87% of patients had maintained their IGA score of 0 or 1 by week 52, and 92% of patients had maintained EASI-75 by the same endpoint.
Regarding patients who had achieved EASI-50 by week 16, 21% to 28% of patients achieved IGA 0/1 by week 52, and 11% to 16% more patients had achieved EASI-75. Over time, responses were described as continuous in their improvement.
Furthermore, an equal to or greater than 5-point reduction in Dermatology Life Quality Index score was achieved in 93.4% (Q2W) and 90.0% (Q4W) of patients by the conclusion of the study.
“We are very pleased with the stage 2 results of our pivotal China trial, showing that the strong improvements observed in patients at week 16 in stage 1 were maintained with both every two weeks and every four weeks dosing regimens,” said Zheng Wei, PhD, in a press release. Wei is the co-founder and CEO of Connect Biopharma.
"In a pre-Thanksgiving Day surprise, Connect BioPharma released its China stage 2 clinical trial results for rademikibart in moderate to severe atopic dermatitis. Rademikibart really distinguished itself among the AD biologics with >80% of patients maintaining both IGA 0/1 and EASI-75 with q4 week dosing, over 10 points better than the next closest biologic. This suggests rademikibart has best in class potential for AD biologics with monthly dosing," said Christopher Bunick, MD, PhD, a Dermatology Times Editorial Advisory Board member and associate professor of dermatology and physician-scientist at the Yale School of Medicine in New Haven, Connecticut.
"Patients receiving rademikibart experienced conjunctivitis at rates ranging from 5.3-8.2% (2.7% placebo), injection site reactions 5.3-9.1% (2.7% placebo), and herpes viral infection 1.8-3.5% (1.8% placebo). Connect Biopharma also announced partnership with Simcere Pharmaceutical to help develop rademikibart for the China market, and will look for partners for a global phase 3 program," Bunick said. "Dermatologists can expect further phase 2b data on rademikibart in asthma in late 2023. This will help identify the potential of rademikibart across similar indications that dupilumab is currently approved for."
Reference
Connect Biopharma Holdings Limited. Connect Biopharma announces positive long-term data from the China pivotal trial of rademikibart in patients with moderate-to-severe atopic dermatitis. GlobeNewswire News Room. November 21, 2023. Accessed November 21, 2023. https://www.globenewswire.com/news-release/2023/11/21/2783837/0/en/Connect-Biopharma-Announces-Positive-Long-Term-Data-from-the-China-Pivotal-Trial-of-Rademikibart-in-Patients-with-Moderate-to-Severe-Atopic-Dermatitis.html
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.












