- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
What's New in Truncal Acne?
Author(s):
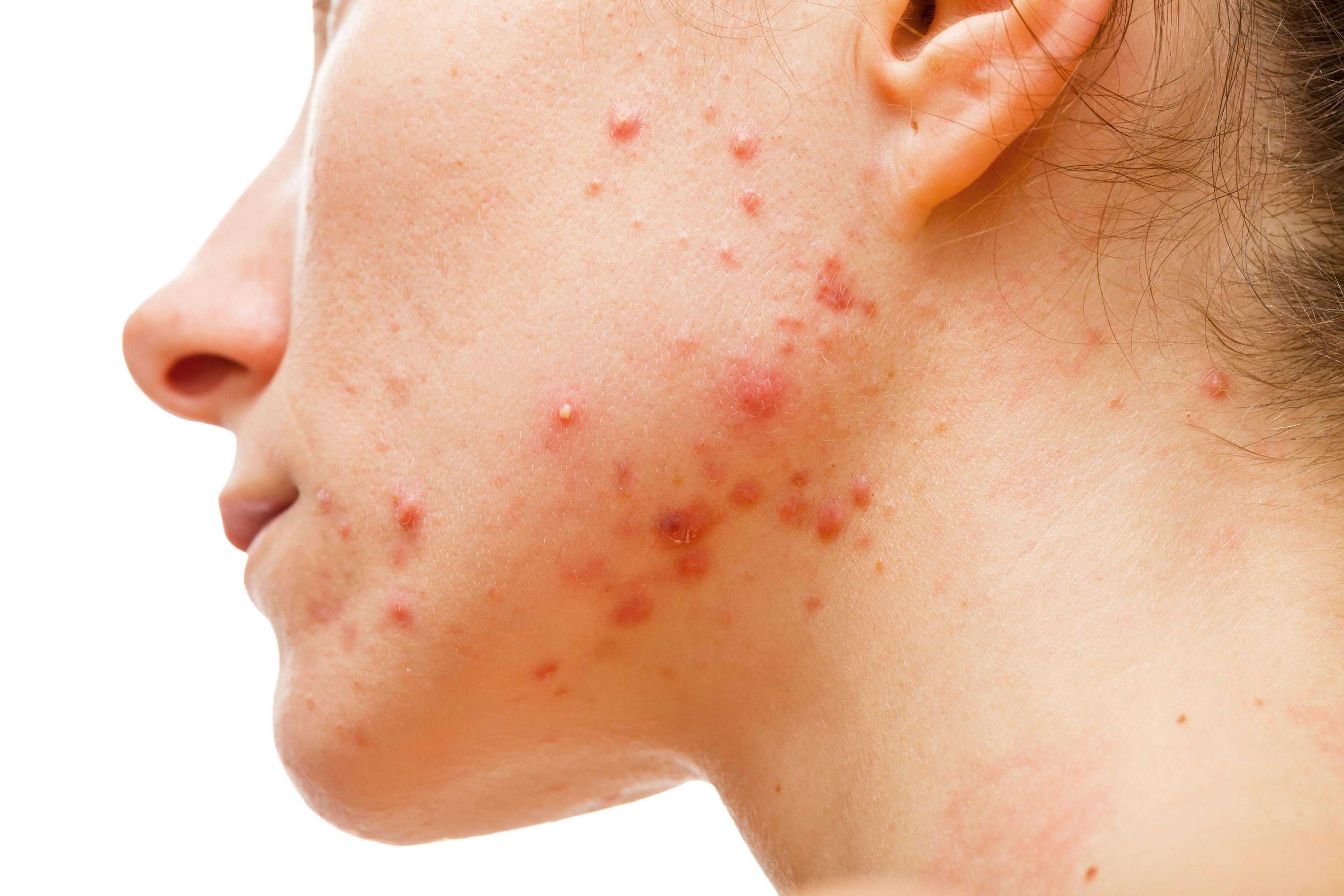
Recent observations highlight new treatment efficacy in women and skin of color patients with truncal acne.
Recent research and clinical experience are expanding dermatologists’ understanding of the role of trifarotene (Aklief; Galderma Laboratories, LP) in truncal acne, particularly in patients with skin of color (SOC). Additionally, a large prospective spironolactone trial is filling knowledge gaps regarding treatment of truncal acne in women.
Truncal acne presents particular problems in patients with SOC, says Andrew F. Alexis, MD, MPH, vice chair for diversity and inclusion in the Department of Dermatology at Weill Cornell Medicine in New York, New York and a member of the editorial advisory board for Dermatology Times®.
Based on his observations, Alexis says not only does postinflammatory hyperpigmentation (PIH) occur more frequently in Fitzpatrick skin types IV to VI , but truncal PIH in these patients is often more severe than facial PIH. “On the trunk, it’s my experience that postinflammatory hyperpigmentation tends to be longer lasting and more difficult to treat,” he says, noting that his comments are based on his own clinical experience rather than the 52-week study.
Barriers to successful treatment include the difficulty of applying topical medicines to the chest and back and a reticence to discuss truncal locations unless a dermatologist specifically asks about them.1 Additionally, Alexis hypothesized that perhaps truncal PIH tends to be more dermal than epidermal or that epidermal turnover rates may be lower on the trunk than on the face.
A recently published journal article by Bell et al noted that in the 52-week phase 3 trial of trifarotene, 66.9% of treated patients with truncal acne achieved Physician Global Assessment (PGA) scores of clear or almost clear.2 Trifarotene-treated patients with truncal acne also achieved significantly higher PGA success rates than did placebo-treated patients, as well as significantly greater reductions in inflammatory and noninflammatory lesion counts at 12 weeks.
“Being the first retinoid to be studied and approved for truncal acne, trifarotene has been a very useful addition to our therapeutic armamentarium, specifically for patients with skin of color,” says Alexis. “I have found it to be very useful for managing both the acne and the postinflammatory hyperpigmentation on the trunk and the face.”
Patients can experience dryness (usually mild to moderate), peeling, and irritation in the first few weeks of use, he notes. “Counseling patients on how to manage those potential adverse events is key, especially for patients with skin of color, because significant irritation can cause more pigmentary alterations including hyperpigmentation.”
Alexis recommends applying the medication thinly in affected areas, reducing dosing to every other night if needed, and applying a noncomedogenic moisturizer immediately after—or for very sensitive skin, before—trifarotene application.
He also reminds dermatologists about the value of oral isotretinoin for patients who do not respond adequately to topical therapies and oral antibiotics. “In my experience, using oral isotretinoin in patients of color with truncal acne produces the faster resolution of the postinflammatory hyperpigmentation as well as the acne,” Alexis says. Although studies document lower isotretinoin prescription rates in African-American patients,3,4 the reasons for this disparity remain unclear, he adds.
To address a dearth of long-term spironolactone data in truncal acne, investigators followed 403 consecutively treated patients for up to 2 years.5 In perhaps the largest single observational study of spironolactone for acne, investigators incorporated quantitative measures not used in prior trials.
“Many studies will assess patients qualitatively—did they get better or not? We wanted to go beyond that and use a validated outcome measure called the Comprehensive Acne Severity Scale (CASS),” says principal author John S. Barbieri, MD, MBA, a postdoctoral research fellow in the Department of Dermatology at Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
Using a 0 to 5 scale, CASS enabled separate assessment of the face, chest, and back before, during, and after treatment. Among patients who had baseline and first follow-up CASS chest and back scores available (n=106 in both areas), 84.0% and 80.2%, respectively, experienced CASS declines or complete clearance. At first follow-up (approximately at 2-3 months of treatment), the proportions of patients with chest acne and back acne who achieved clearance were 77.4% and 67.9%, respectively.5
“I personally believe the optimal dose is around 100 to 150 mg daily for most patients. Our data support that,” Barbieri says.
The main drawback of higher doses is that they carry the potential for more adverse effects, according to Barbieri. “But we know that higher doses tend to have higher rates of clearance and improvement,” he says. Rather than starting at lower doses, Barbieri suggests starting at 100 mg to 150 mg daily and reducing dosage if patients experience adverse effects or clearing.
“Patients want to get better quickly. Since spironolactone can take several months to reach peak effectiveness, if you start at a low dose and the patient doesn’t have enough improvement, now you are several months behind,” he says. “Our data suggest that it is much more common to increase the dose to 100 to 150 mg per day than to decrease to lower doses.”
Alexis notes that more research is needed. “The 52-week study did not include a large SOC population, so additional studies with greater representation of SOC patients are warranted,” he says. “At this time, I’m not aware of any studies that describe the greater persistence of postinflammatory hyperpigmentation on the trunk versus the face. But this is an area where I and some colleagues who have expertise in acne and skin of color are working on adding more knowledge.”
Disclosures:
Alexis has been a consultant, advisor, or investigator for multiple companies that market acne treatments including Galderma Laboratories, LP, the maker of trifarotene. Barbieri reported no relevant or financial interests.
References:
1. Del Rosso JQ, Stein-Gold L, Lynde C, Tanghetti E, Alexis AF. Truncal acne: a neglected entity. J Drugs Dermatol. 2019;18(12):205-1208.
2. Bell KA, Brumfiel CM, Haidari W, Boger L. Trifarotene for the treatment of facial and truncal acne. Ann Pharmacother. 2021;55(1):111-116.doi:10.1177/1060028020934892
3. Bell MA, Whang KA, Thomas J, Aguh C, Kwatra SG. Racial and ethnic disparities in access to emerging and frontline therapies in common dermatological conditions: a cross-sectional study. J Natl Med Assoc. 2020;112(6):650-653. doi:10.1016/j.jnma.2020.06.009
4. Barbieri JS, Shin DB, Wang S, Margolis DJ, Takeshita J. Association of race/ethnicity and sex with differences in health care use and treatment for acne. JAMA Dermatol. 2020;156(3):312-319. doi:10.1001/jamadermatol.2019.4818
5. Garg V, Choi JK, James WD, Barbieri JS. Long-term use of spironolactone for acne in women: a case series of 403 patients. J Am Acad Dermatol. Published online January 9, 2021. doi:10.1016/j.jaad.2020.12.071

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.