- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
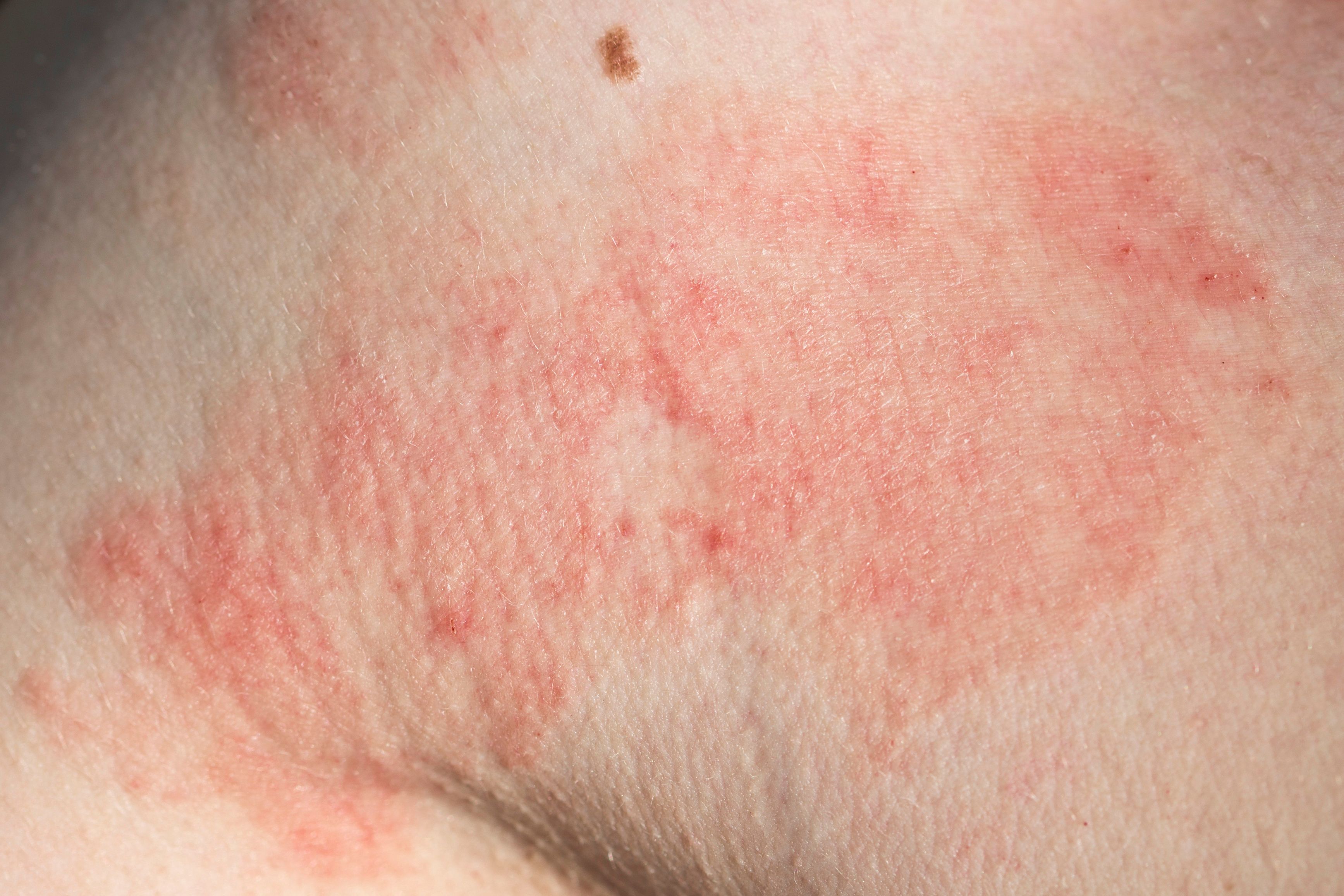
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
5-Year Efficacy Data of Dupilumab in Adults With Moderate to Severe AD
Author(s):
Dupilumab’s efficacy was sustained through the 260-week open-label study.
A poster presentation from Maui Derm NP & PA Fall in Asheville, North Carolina, investigated the efficacy of dupilumab in a long-term, 5-year open-label study for the treatment of adults with moderate to severe atopic dermatitis (AD). According to the study authors, topical therapies are often insufficient for controlling moderate to severe AD, and systemic immunosuppressants have safety concerns for long-term use. Dupilumab, a fully human monoclonal antibody that targets interleukin (IL)-4 and IL-13 receptors, offers a new approach to managing AD. LIBERTY AD OLE (NCT01949311) aimed to evaluate the long-term efficacy and safety of dupilumab over a 5-year period in adult patients with moderate to severe AD.1
Adults aged 18 and above who had participated in any of the earlier dupilumab studies (ranging from phase 1 to phase 3) were enrolled in the open-label extension (OLE) study. Initially, patients were treated with 300mg of dupilumab weekly, and in 2019, some patients transitioned to a bi-weekly regimen. Concomitant treatments such as topical corticosteroids and calcineurin inhibitors were allowed. The data was collected from 2677 patients.
Out of the 2677 patients who enrolled in the study, 2207 completed treatment up to week 52, 362 up to week 172, and 334 up to week 260. The most common reason for study withdrawal was the approval and commercialization of dupilumab in the patient's home country. Only a small percentage (1.9%) withdrew due to lack of efficacy. By the end of the study, 88.9% of patients achieved a 75% reduction in the Eczema Area and Severity Index (EASI) score from the baseline of the parent study, and 76.2% achieved a 90% reduction in the EASI score. At week 260, 66.5% of patients achieved a ≥4-point reduction in the Peak Pruritus Numerical Rating Scale (PP-NRS) score from the parent study baseline.
Over the course of the study, 2276 patients (85.0%) reported treatment-emergent adverse events. Importantly, only 3.8% of patients discontinued treatment permanently due to adverse events, indicating an acceptable safety profile for dupilumab.
According to the study authors, this 5-year open-label study reaffirms the long-term efficacy and safety of dupilumab in adult patients with moderate to severe AD. The majority of patients experienced substantial improvements in AD signs and symptoms, including skin lesions and pruritus, as demonstrated by the reduction in EASI scores and the PP-NRS score. The safety profile of dupilumab remained consistent with the safety observed in earlier placebo-controlled studies of dupilumab.
The data from this study supports the use of dupilumab as a valuable addition to the therapeutic options available for the management of moderate to severe AD.
Reference
- Beck A, Bissonnette R, Deleuran M, et al. Long-term efficacy of dupilumab in adults with moderate-to-severe atopic dermatitis: results from a 5-year open-label extension trial. Presented at: Maui Derm NP & PA Fall; September 27-30, 2023; Asheville, NC.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.