- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Artificial Intelligence: Friend or Foe?
This month's cover feature delves into the new applications and use of AI in dermatology and considers how AI can support clinicians rather than hinder them.
Image courtesy of OM1
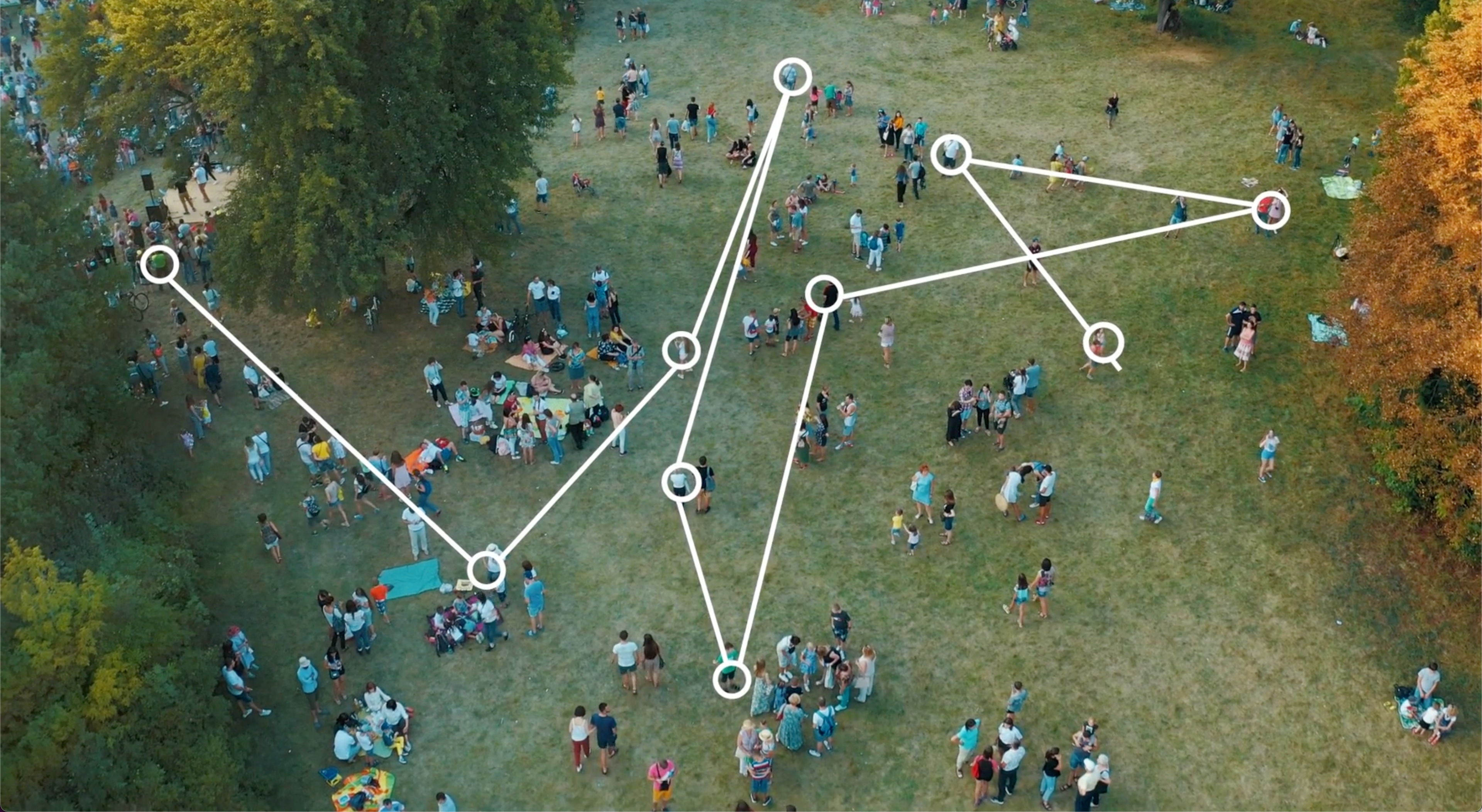
Artificial intelligence (AI) has been a buzzword in health care for some time, but do clinicians, especially in dermatology, view it as friend or foe? Applications and use of AI in dermatology are comparatively new and have focused primarily on the relatively narrow application of analyzing images of skin disease. This limited uptake began, ironically, because image analysis seemed like relatively low-hanging fruit, with the promise of improving workflows and efficiency.
Experience has shown that real value is challenging to capture at scale, largely because skin image data vary in quality, completeness, and representativeness, often leading to unreliable results. Additionally, prioritization of focus remains challenging. A single, unified ability to assist in diagnosing all skin diseases has not yet been developed, and selecting which use cases create most value in the meantime has been a complex task without many obvious quick wins.
Beyond a Buzzword
The field continues to advance rapidly, however, and has shown exciting promise within skin cancer, atopic dermatitis, and psoriasis. AI can aid in improving the screening process for certain skin cancers, identify patients with atopic dermatitis, and assist in personalizing treatment decisions for patients with psoriasis. The key word here is assist: AI can be a helpful tool, but clinical oversight is still needed for confirmed diagnosis, proper treatment decisions, and appropriate follow-up.
Harnessing the power of AI to find patients, especially those living with undiagnosed inflammatory conditions, is an especially exciting application because patients who might otherwise go untreated could reach a diagnosis and appropriate treatment plan. Traditionally, even though we know patients’ medical records provide tremendous insight into how their conditions evolve over time, much of that richness has been inaccessible. AI and machine learning have allowed us to identify patterns within these real-world data at scale, associated with outcomes we care about—presence of a certain condition, for example, or risk of rapid progression. With this information, we can assemble a “digital fingerprint” for patients we want to understand better, and then use that fingerprint to analyze patient records in new data sources.
This approach lets us make personalized medicine—another buzzword—a real-world option for patients’ clinical care. By using AI tools, we can apply what we learn from vast data sets covering millions of patients to better understand a single patient’s health history. With this ability to translate from learning at scale to personalization for the individual, clinicians can summon a range of insights that can help in decision-making. They can order digital assessment panels for multiple outcomes at once, using their patient’s health history records, to assist with diagnostic evaluations, predictions about treatment efficacy, and even assessment for potential participation in clinical trials. For life science companies, this personalization helps highlight patients who could benefit most from available treatments, and spotlights areas of greatest unmet need for future development.
AI’s Role in Treatment Plans

AI, coupled with the power of real-world data, can also assist clinicians in optimizing treatment plans for their patients. With many advanced options now available for atopic dermatitis, for example, selection around both efficacy and safety is of utmost importance. Purpose-built, specialized data networks around specific conditions form a foundation for measuring quality of care and clinical outcomes, and ultimately for AI model training. These data sets are currently relatively underutilized and can provide powerful insight into how patients’s diseases are managed in practice. Using AI-based personalization, these insights can be translated to the individual. For example, a patient clinically similar to others who did not respond to a particular treatment may also be less likely to respond to the treatment. These similarities may not be obvious, but by isolating them, AI can surface this similarity and alternate treatments can be considered.
Purpose-built networks gathering data on patients, treatments, and outcomes in an automated way also allow us to evaluate effectiveness, better understand the natural history of disease, and monitor for safety signals as longitudinal patient journeys develop. Using patient-centric tools, such as electronic patient-reported outcomes (ePRO) platforms, as part of these networks allows direct data collection from patients—a key advantage over waiting for infrequent clinical visits for updates on patient status. Also, ePROs add further depth around outcomes and events not typically available in electronic medical record data. Linked to the broader data sets and collected prospectively, ePROs provide unparalleled insight into what is actually happening to patients in the real world outside clinical care settings.
FDA Weighs In on Data Use

As focus is shifting to practical and actionable use of real-world data, the US Food and Drug Administration (FDA) issued a final guidance in August 2023 for industry in relation to clinical study designs that use real-world data (RWD). The guidance outlines the agency’s expectations for sponsors submitting new drug applications or biologics license applications using RWD to support the safety or effectiveness of a drug in clinical studies that are not subject to 21 CFR part 312.1
For observational studies, the guidance outlines the agency’s general expectations for study conduct. This guidance is meant to aid stakeholders in following FDA requirements, and to ensure that the agency can appropriately evaluate observational studies that are submitted in marketing applications to support the safety and effectiveness of a medication. This guidance is part of a series of guidance’s that accompany the agency’s RWE program2 and in support of the 21st Century Cures Act and the Prescription Drug User Fee Act.
Looking Ahead
As we’ve discussed, the hype around AI is finally translating into real-world value, and we are seeing broader adoption of real-world data. With new guidance from the FDA, we have a clearer path to personalized medicine and AI-powered clinical decision support in dermatology. At their core, these advances use subtle patterns observed at scales never before possible to provide meaningful insights for individual dermatology patients. Implemented well, these tools can be seamlessly integrated into existing workflows, facilitating communication among technical experts, clinicians, and the patient. The ultimate goal is not to use AI for its own sake but to further our common goal of getting the right treatment to the right patient at the right time.
With specialization in chronic conditions such as alopecia areata, atopic dermatitis, hidradenitis suppurativa, prurigo nodularis, psoriasis, psoriatic arthritis, and vitiligo, OM1 is reimagining real-world data and evidence. It is doing so by developing large, electronically connected networks of clinicians and health data in immunology; cardiometabolic; mental health and neurosciences; and respiratory and ear, nose, and throat specialty areas. Leveraging its extensive clinical networks and an unparalleled technology and AI platform (PhenOM), OM1 offers industry-leading enriched health care data sets, research analytics, data modeling, insights reports, and retrospective and prospective clinical studies. With a focus on high-quality data and clinical outcomes, the offerings are used for accelerating research, demonstrating treatment effectiveness, supporting regulatory submissions, monitoring safety, and informing commercialization.
Stefan Weiss, MD, FAAD

Joseph Zabinski, PhD, MEM

Stefan Weiss, MD, FAAD, is the managing director of dermatology at OM1.
Joseph Zabinski, PhD, MEM, is the managing director of AI and personalized medicine at OM1.
OM1 is a real-world data, AI and technology company with a focus on chronic diseases that is actively developing large electronically connected networks of clinicians and health data in dermatology and other specialty areas.
References
1. Considerations for the use of real-world data and real-world evidence to support regulatory decision-making for drug and biological products. FDA. Updated August 30, 2023. Accessed September 8, 2023. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/considerations-use-real-world-data-and-real-world-evidence-support-regulatory-decision-making-drug?utm_medium=email&utm_source=govdelivery
2. FDA. Framework for FDA’s real-world evidence program. December 2018. Accessed September 8, 2023. https://www.fda.gov/media/120060/download?utm_medium=email&utm_source=govdelivery

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.