- Case-Based Roundtable
- General Dermatology
- Eczema
- Chronic Hand Eczema
- Alopecia
- Aesthetics
- Vitiligo
- COVID-19
- Actinic Keratosis
- Precision Medicine and Biologics
- Rare Disease
- Wound Care
- Rosacea
- Psoriasis
- Psoriatic Arthritis
- Atopic Dermatitis
- Melasma
- NP and PA
- Skin Cancer
- Hidradenitis Suppurativa
- Drug Watch
- Pigmentary Disorders
- Acne
- Pediatric Dermatology
- Practice Management
- Prurigo Nodularis
- Buy-and-Bill
Article
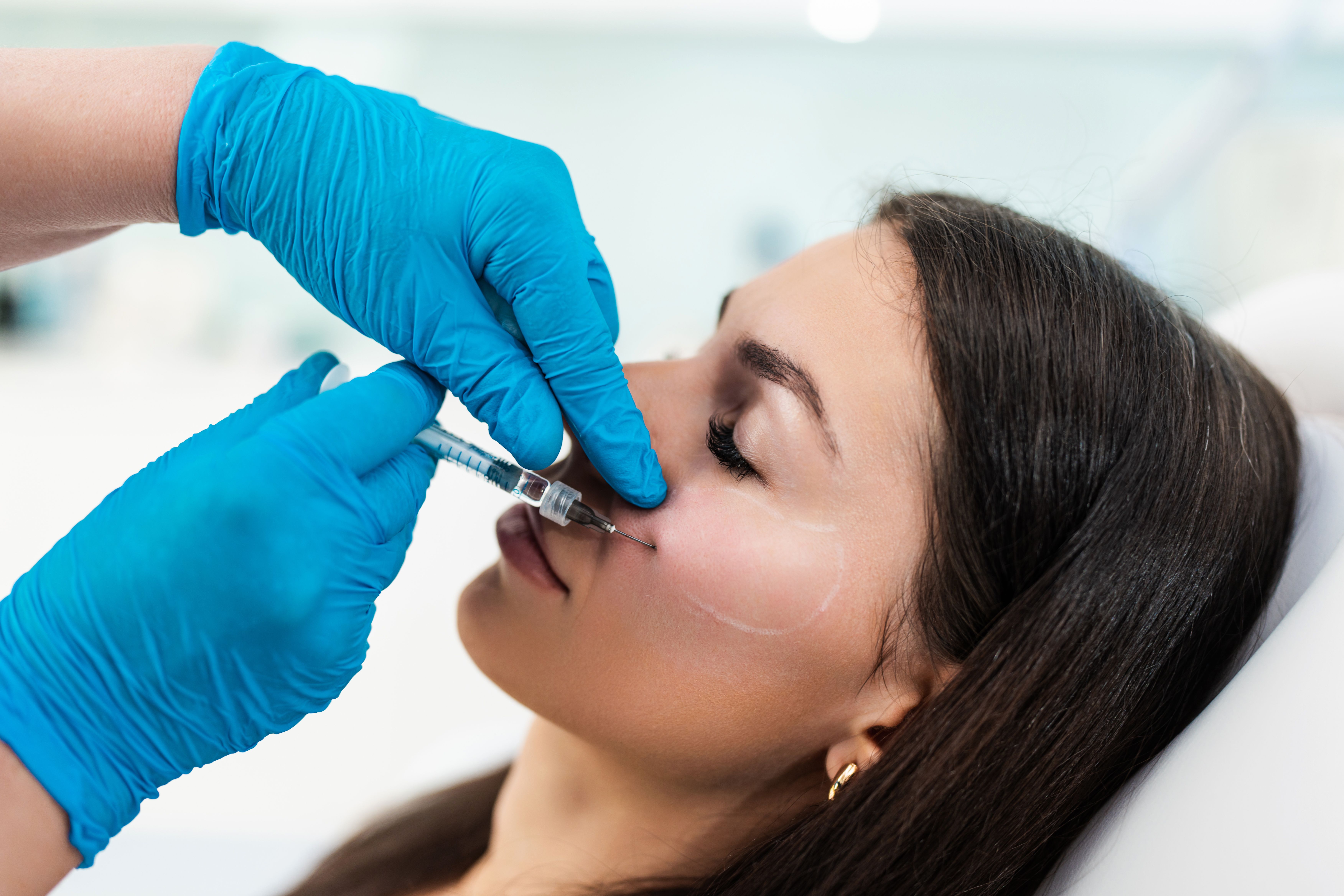
Botulinum toxin injections found effective for facial wound-healing procedures
Rochester, Minn. - Results of a Mayo Clinic study show that treating a facial wound in the early healing phase with botulinum toxin improves the appearance of a scar later.
Rochester, Minn. - Results of a Mayo Clinic study show that treating a facial wound in the early healing phase with botulinum toxin improves the appearance of a scar later.
The findings appear in the August issue of Mayo Clinic Proceedings.
The researchers found that an injection of botulinum toxin early after the occurrence of a wound, skin cancer biopsy or removal will paralyze the region, thus creating a smooth surface in which the wound can heal. The procedure prevents muscle movement from wrinkling the wound site, allowing for a flat surface for healing and leaving a smoother final scar. The same process also could work if an unsightly older scar is surgically removed and botulinum toxin injected into the wound at the time of the scar revision surgery.
According to the study, side effects of the botulinum toxin injections were minimal: an occasional small bruise at the injection site or a headache. The largest potential risk in injecting botulinum toxin in the facial area would be transient paralysis of an important function.
The study cautions that although injections with botulinum toxin would be available at local physicians’ offices, the Food and Drug Administration (FDA) has not approved botulinum toxin injections for wound healing use. The study adds that the next step in the research would be to conduct a phase 3 trial to determine the appropriate dosage of the botulinum toxin; discover whether the injections are useful for better healing of scars elsewhere on the body and to submit more findings to the FDA for its consideration and eventual approval.