- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Opinion
Video
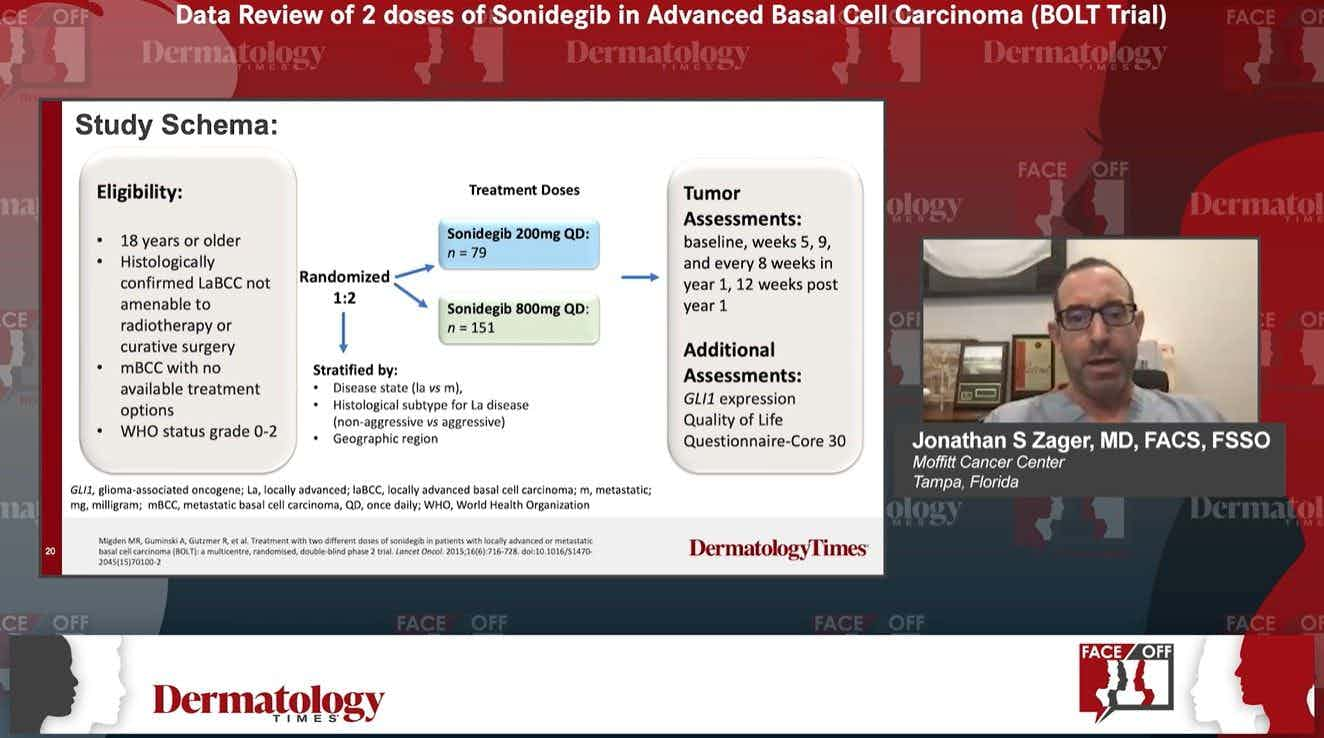
Data Review of the Efficacy and Safety of Vismodegib in Advanced Basal Cell Carcinoma (EVRIANCE Trial)
Author(s):
Lilia Correa-Selm, MD, FAAD, FACMS, reviews the clinical trial data relating to the EVRIANCE study examining the use of vismodegib treatment in patients with locally advanced basal cell carcinoma, highlighting key efficacy and safety endpoints.
Jonathan S Zager, MD, FACS, FSSO
Moffitt Cancer Center
Tampa, Florida
Lilia Correa-Selm, MD, FAAD, FACMS
Moffitt Cancer Center
Tampa, Florida
Aaron Farberg, MD
Bare Dermatology
Dallas, Texas
John Strasswimmer, MD, PhD
Florida Atlantic University
Delray Beach, Florida
Andrew Weinstein, MD, MPH, FAAD
Boynton Beach Skin, University of Miami
Boynton Beach and Coral Gables, Florida
Series Description: Experts in dermatology discuss the clinical trial data from the BOLT and EVRIANCE clinical trials and review patient cases related to the management of patients with locally advanced basal cell carcinoma treated with sonidegib and vismodegib.
Segment Description: Lilia Correa-Selm, MD, FAAD, FACMS, reviews the clinical trial data relating to the EVRIANCE study examining the use of vismodegib treatment in patients with locally advanced basal cell carcinoma, highlighting key efficacy and safety endpoints.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.