- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
FDA accepts NDA filing for topical papulopustular rosacea treatment
Author(s):
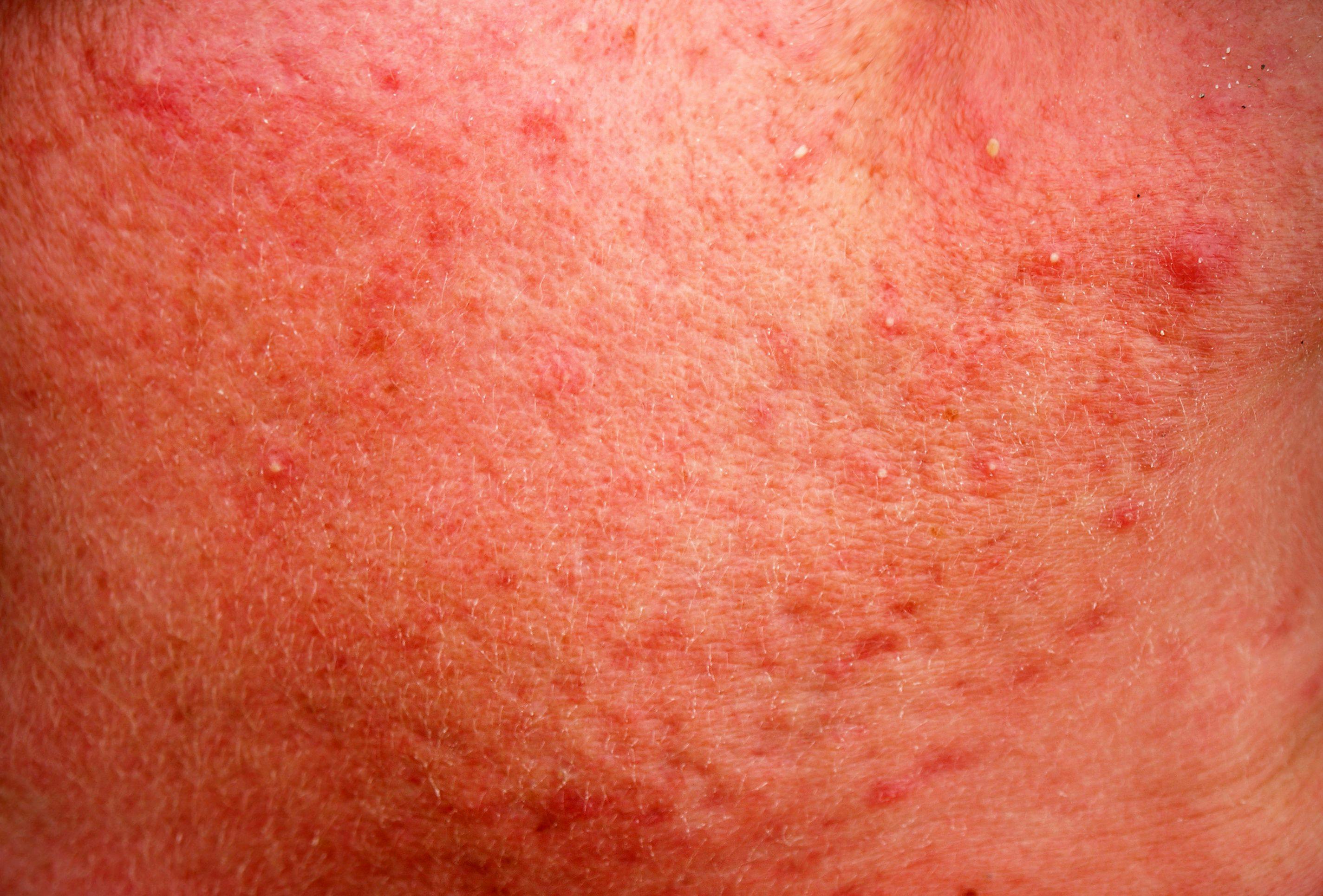
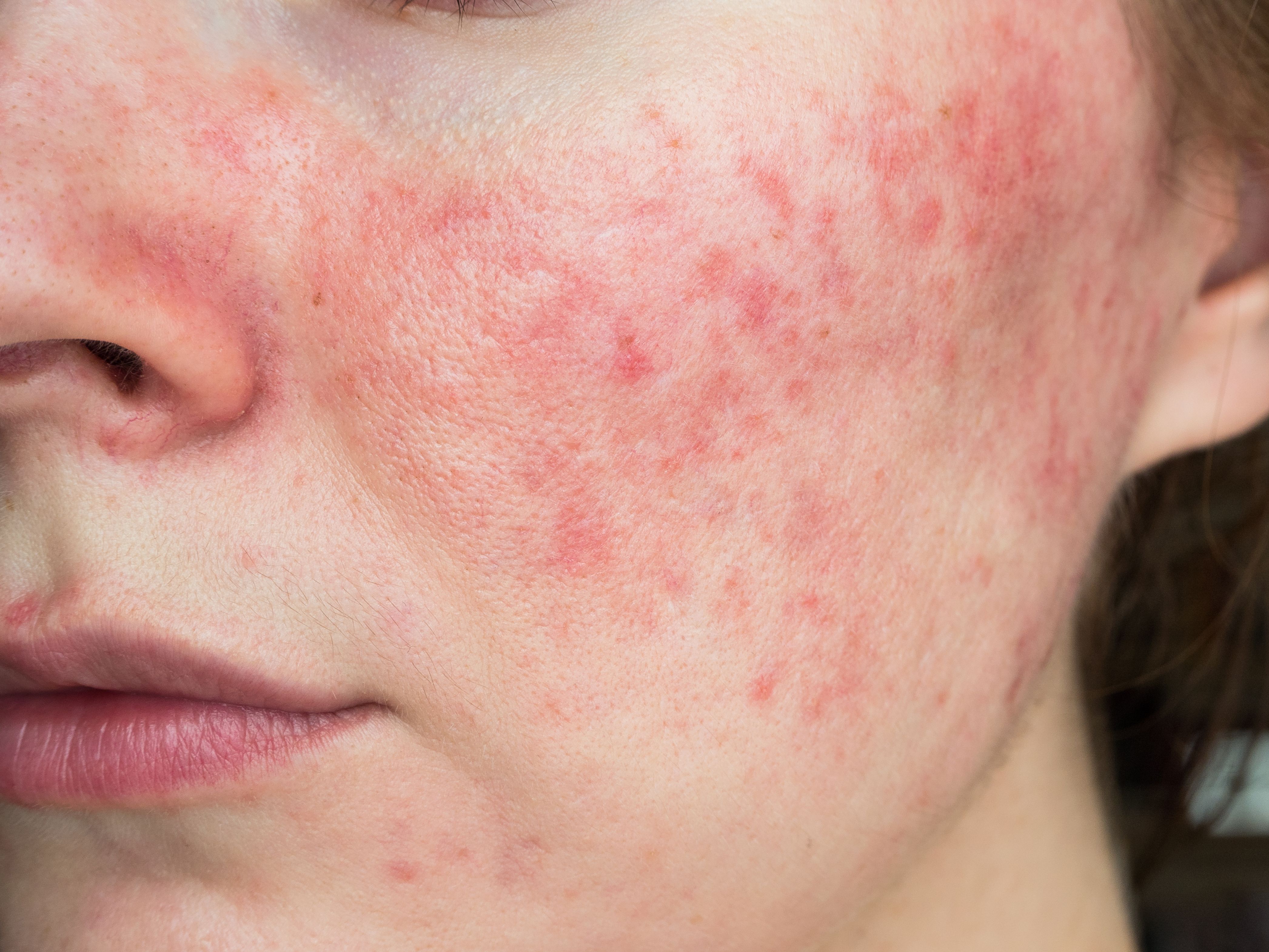
The U.S. Food and Drug Administration (FDA) has accepted the New Drug Application (NDA) filing for Sol-Gel’s Epsolay, a 5% microencapsulated benzoyl peroxide cream for the treatment of inflammatory lesions of rosacea or papulopustular rosacea.
Sol-Gel Technologies announced the U.S. Food and Drug Administration (FDA) has accepted its New Drug Application (NDA) filing for Epsolay (benzoyl peroxide). If approved, Epsolay has the chance to be the first single-agent benzoyl peroxide prescription drug approved by the FDA.1
Epsolay is a topical 5% encapsulated benzoyl peroxide cream intended to treat the inflammatory lesions of rosacea (papulopustular rosacea or PPR). Benzoyl peroxide is not currently approved by the FDA to treat rosacea and is known to potentially cause skin irritation, including stinging, burning and erythema in individuals with PPR.2
Sol Gel’s patented microencapsulation technology aims to reduce this risk by encapsulating the benzoyl peroxide inside porous silica microcapsules, according to the company. This generates a barrier between the skin and the drug - with the microcapsules dispensing doses of the drug while the barrier reduces benzoyl peroxide’s oxidative effect, which can cause skin irritation. 2
MORE: Sol-Gel’s rosacea cream may offer patient paid relief
The filing’s acceptance is backed by positive data from two phase 3, identical, randomized, multicenter, double-blind, 12-week trials investigating the safety and efficacy of Epsolay versus vehicle in patients with PPR.
In both trials, the benzoyl peroxide topical demonstrated a statistically significant improvement in both co-primary endpoints, including a) a proportion of patients achieving “clear” or “almost clear” according to the Investigator Global Assessment (IGA) scale and b) an absolute mean reduction from baseline in inflammatory lesion count beginning as early as week 2 and held through week 12.1
RELATED: Sol-Gel pipeline update 2020
Additionally, Epsolay showed a favorable safety and tolerability profile comparable to vehicle. The most common adverse reactions occurring in the application were mild-to-moderate pain, erythema and pruritus, all of which were seen in >1% of Epsolay patients. 1
“Papulopustular rosacea is a chronic and recurrent inflammatory skin disorder that affects nearly 5 million Americans. Regretfully, rosacea patients are dissatisfied with the efficacy of current therapies,” says Alon Seri-Levy, M.D., CEO of Sol-Gel. “The results from our phase 3 studies showed statistically significant higher success in IGA compared with the vehicle, at every visit, and as early as at week 2, as well as statistically significant higher reduction in absolute inflammatory lesion counts compared with the vehicle, at every visit, and as early as week 2. In addition, a quarter of Epsolay patients in both trials reached their treatment goals within a month, which is very encouraging.”
The Prescription Drug User Fee Act (PDUFA) date for the FDA to make a decision on Epsolay is set for April 26, 2021.1
References:
1. Sol-Gel Technologies Announces FDA Acceptance for Filing of New Drug Application for Epsolay® for the Treatment of Inflammatory Lesions of Rosacea: Sol-Gel. (2020, September 10). Retrieved September 14, 2020, from https://ir.sol-gel.com/news-releases/news-release-details/sol-gel-technologies-announces-fda-acceptance-filing-new-drug
2. Petronelli, M. (2020, March 3). Sol-Gel's rosacea cream may offer patients rapid relief. Retrieved September 14, 2020, from https://www.dermatologytimes.com/view/sol-gels-rosacea-cream-may-offer-patients-rapid-relief

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.