- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
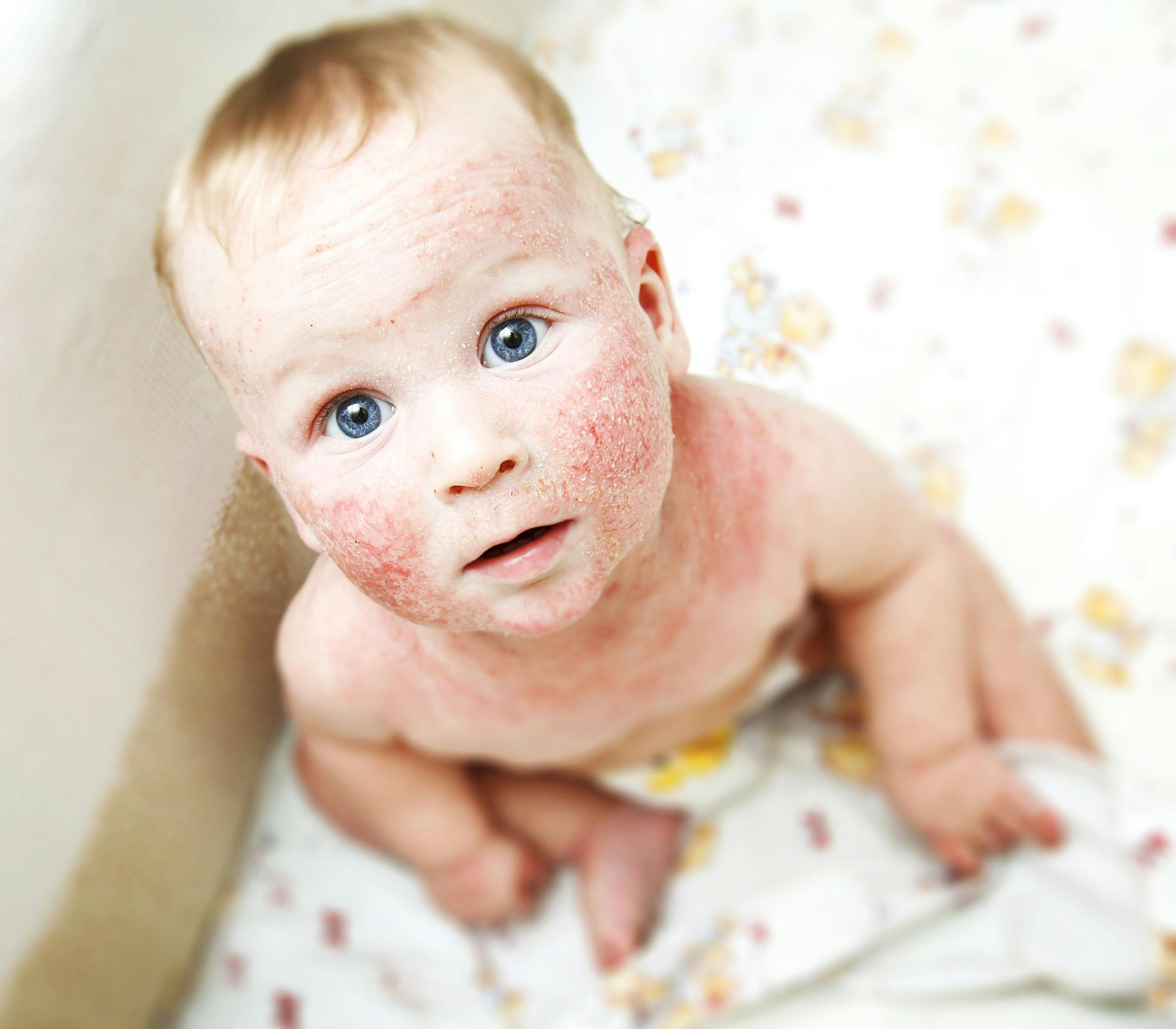
FDA says start with pediatric patients in atopic dermatitis trials
Author(s):
The FDA has reversed a long-standing guidance stating that sponsors of clinical trials for atopic dermatitis (AD) in pediatrics need not start the trial in adults first. Here's what you need to know.
The FDA announced in October that it has reversed a long-standing guidance stating that sponsors of clinical trials for atopic dermatitis (AD) in pediatrics need not start the trial in adults first. (©Skylines/Shutterstock.com)
The FDA has reversed a long-standing guidance stating that sponsors of clinical trials for atopic dermatitis (AD) in pediatrics need not start the trial in adults first. The decision, which was announced in October, was influenced by recommendations from its Dermatologic and Ophthalmic Drug Advisory Committee.
There are some caveats:
- Upon approval, applicants should disclose how to use the drug safely and effectively in pediatric patients.
- AD trials should be initiated early in development once efficacy and safety data are available.
- Safety questions, such as the risk for longlatency or low-frequency adverse reactions, do not have to be resolved before initiation of studies in pediatric patients with AD.
- It is not necessary to have an extensive safety database in adults before starting pediatric atopic dermatitis clinical trials due to the extent of disease-related morbidity in children, the high risk of disease-related progression in this population, and the relative risk-benefit calculus with off-label use of immunosuppressive therapies.
- All pediatric age groups should be studied, including children two years old and younger. However, it may be necessary to first have safety outcomes from trials conducted in older pediatric patients; resolve age-related technical issues; and, address any potential safety-related concerns.
The guidance, “Atopic Dermatitis: Timing of Pediatric Studies During Development of Systemic Drugs Guidance for Industry,” is online at www.fdanews.com/10-02-18-AtopicDermatitis.pdf.

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.