- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Hidradenitis Suppurativa: Current and Future Treatments
Author(s):
Hidradenitis suppurativa is a chronic inflammatory skin condition that presents with painful, deep, and inflamed lesions found in flexural, apocrine gland-bearing sites.
Introduction
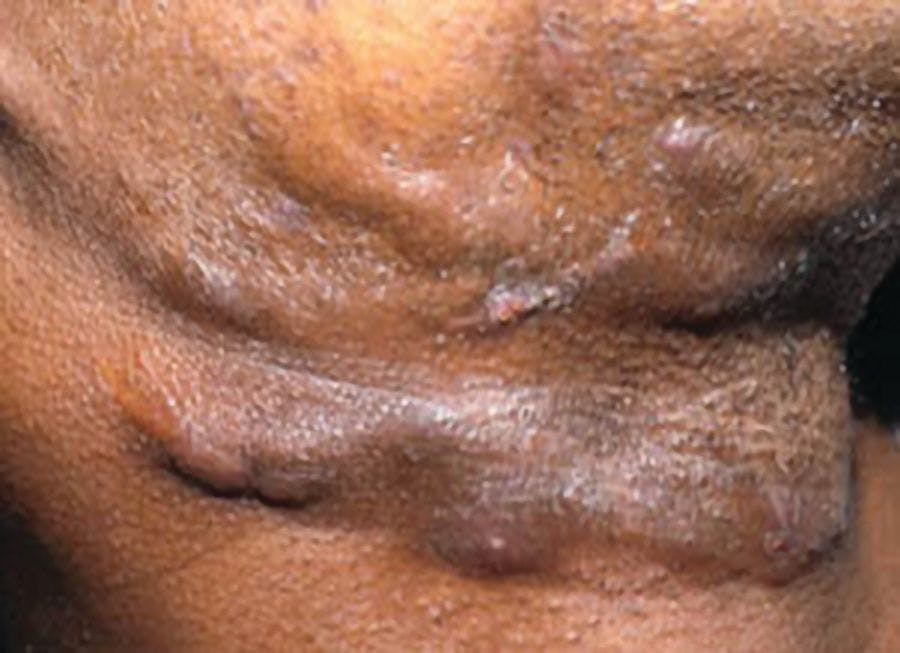
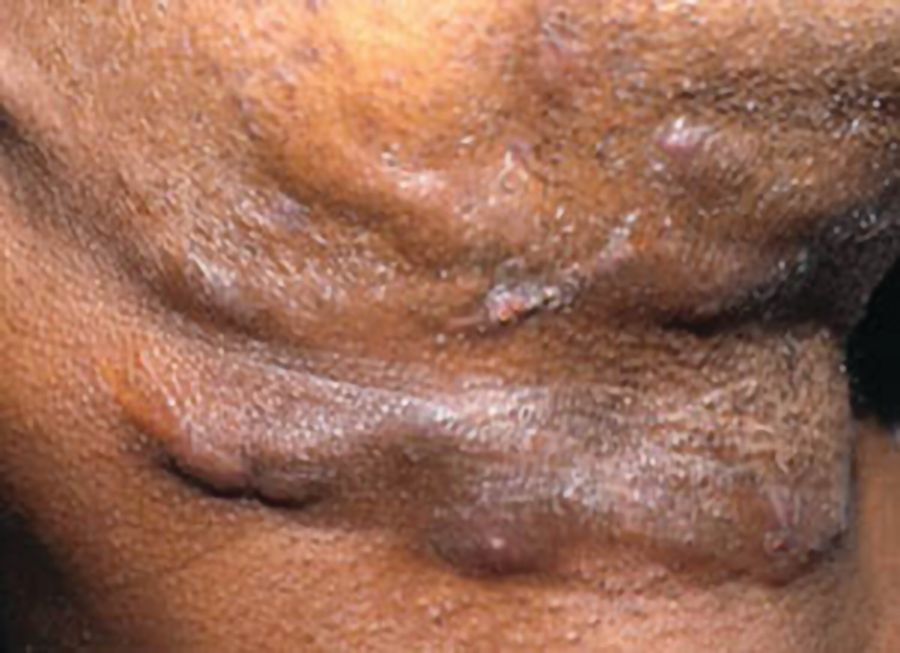
Hidradenitis suppurativa (HS) is a chronic inflammatory skin condition that presents with painful, deep, and inflamed lesions found in flexural, apocrine gland-bearing sites, especially the axillary, inguinal, and genital areas (Figure 1). In severe cases, sinus tract or skin fistula formation is common.1 The prevalence of HS in patients in North America and Europe ranges from 0.05% to 4.1% in studies from 1996 to 2018, most likely because of differing populations studied and methodologies/criteria used, and the prevalence also varies by race/ethnicity. In addition, the condition is often undiagnosed, particularly in children who account for 2% of cases.2 Studies from 1988 to 2014 found a similar prevalence range of 0.03% to 4.10%; however, 1 study found a higher prevalence of 8%.3
Figure 1: Axilla of adolescent with HS
Though the pathogenesis of HS is still under investigation, HS is known to be a multifactorial disease, with genetic, environmental, and immunological factors involved. These factors lead to immune activation around hair follicles and hyperkeratosis, leading to follicular blockage. Bacterial propagation, especially within the blocked follicles, promotes further immune activation. Immune cells, such as macrophages, TH1 cells, and TH17 cells, secrete pro-inflammatory cytokines including tumor necrosis factor (TNF), IL-1β, and IL-17. This activates tissue cells, encouraging additional immune cell infiltration and inflammation, which eventually leads to pus formation, tissue destruction, and scarring.4 This inflammation can also affect other organs, and HS is associated with metabolic syndrome, diabetes, inflammatory bowel disease, atherosclerosis, and spondyloarthropathies. Patients with HS also experience depression due to negative effects on their personal and professional lives.4
To understand the treatments of HS, it is important to be familiar with the severity scoring system of HS. The Hurley score is used to determine the severity of HS. Hurley I disease presents with 1 or more isolated nodules and abscesses, but no sinus tracts or scarring. Hurley II disease presents with recurrent abscesses with at least 1 sinus tract and scarring; the lesions are separated by normal areas of skin. In Hurley III disease, there are several lesions forming inflammatory plaques, with extensive sinus tracts and scarring that involves the whole anatomical site.5 Another disease severity scoring system is the Sartorius score, which takes into account the anatomic regions affected, the number and type of lesions, the distance between lesions, and whether lesions are separated by normal skin.6 HiSCR, or the HS clinical response, is the most validated and used measure in research to assess treatment response. It is achieved with at least a 50% reduction in the number of inflammatory lesions (abscesses and inflammatory nodules) and no increase in the number of draining fistulas.5
Current Treatments
Given its multifactorial etiology, treatment of HS requires a multifaceted approach. Current treatment options are discussed below.
Lifestyle Changes
HS is associated with obesity, with a positive correlation between disease severity and body mass index (BMI). A retrospective study of patients with HS who underwent bariatric surgery found that over half of patients reported no symptoms or decreased disease activity post-surgery, 20% reported no changes, and 11% reported more activity.6
Smoking is a risk factor for HS, and patients who smoke have a poorer long-term prognosis than patients who never smoked or quit, with fewer achieving remission.6
Topical Treatments
For mild HS (Hurley stage I-II), topical treatments include resorcinol cream and topical clindamycin lotion. Resorcinol cream has both antiseptic and keratolytic properties and patients have been shown to have decreased pain from inflammatory nodules and clinical resolution of lesions. Use of clindamycin has been shown to reduce the number of inflammatory skin lesions and pain after 3 to 4 months.6 Use of topical sodium fusidate ointment with antibacterial soap led to complete healing in a study with patients with Hurley I disease.7
Systemic Treatments
Treatment of moderate to severe HS often requires systemic treatment with oral antibiotics, which both treat infections and provide an anti-inflammatory effect. However, an important consideration when using oral antibiotics in HS treatment is antibiotic resistance. Studies have shown that patients with HS exhibit resistance to several antibiotics.7
Tetracyclines, such as doxycycline and minocycline, inhibit neutrophil migration and chemotaxis, inhibit angiogenesis, and upregulate anti-inflammatory IL-10. One small, randomized, controlled trial (RCT) found that after 16 weeks, oral tetracyclines resulted in a significant reduction in pain and the number of abscess and boils. However, the benefit was comparable to use of topical clindamycin lotion.6
Clindamycin and rifampin together have been shown to be effective in eliminating superinfection and modulating the immune system. Clindamycin enhances chemotaxis and increases the secretion of TNF-α and IL-6. Rifampin suppresses T-cell function, inhibits secretion of IL-1β and TNF- α, and increases secretion of IL-6 and IL-10. Many patients have shown complete remission or at least partial response, with few showing no response or worsening symptoms.6 An alternative is a combination of rifampin, moxifloxacin, and metronidazole, which has also been shown to result in complete remission for many patients.6
In severe HS, intravenous (IV) antibiotics may be helpful. One study found that patients’ Sartorius score decreased after 6 weeks of ertapenem and continued to decrease during the antibiotic consolidation phase that lasted 6 months.6 However, IV antibiotics and rifampin-moxifloxacin-metronidazole are third-line treatments for HS.5
Steroids also have anti-inflammatory effects, and a small study investigating prednisone as an adjunct treatment found that most patients achieved remission, maintained remission post-prednisone, or improved without remission.7 In another study, intralesional corticosteroid therapy reduced swelling, size, redness, suppuration, and pain, possibly because of inhibition of leukotriene synthesis and synthesis of proinflammatory cytokines. However, another RCT found no significant difference between the treatment (intralesional triamcinolone) and placebo arms.7
Oral retinoids, such as acitretin, isotretinoin, and alitretinoin, may alter IL-6 and IFN-γ activity and have antiproliferative activity in keratinocytes, but their efficacy in HS has mixed evidence, with some studies showing good clinical improvement but lower response rates and higher recurrence rates. They may be better in mild disease and are second- or third-line therapy for HS.5
Antidiabetic medications, particularly metformin, inhibit proliferation of proinflammatory cytokines, decrease hepatic glucose production, and improve insulin sensitivity, which reduces inflammation and may affect the expression of genes involved in HS. One study found a reduction in Sartorius scores, while other studies have had mixed results. Glucagon-like peptide-1 analogues such as liraglutide may also be useful in HS treatment.7
Zinc glutamate plays a regulatory role in innate and adaptive immunity, but in a small pilot study relapses were common with dose reductions.7
Hormonal Treatments
Hormonal treatments have also been used to treat HS, since HS improves during pregnancy, worsens during perimenopause, and may occur during postmenopause. While randomized, controlled trials have not looked at hormonal treatment as an alternative or adjunctive therapy with antibiotics or biologics, ethinylestradiol/noregestrel, ethinylestradiol/cyproterone acetate, spironolactone (mineralocorticoid receptor inhibitor), and finasteride (5α-reductase inhibitor) have been used in some patients. Some studies have found improvement with hormonal treatments but recurrence is common after treatment ends.5
Surgery
For mild HS, deroofing or laser treatment may help alleviate symptoms. Incision and drainage may be used acutely for patients with painful and fluctuant abscesses, though recurrence rates after incision and drainage are close to 100%.7,5 Deroofing is associated with lower recurrence rates, and deroofing combined with sinus tract excisions results in better improvement and decreased recurrence rates.5 For severe disease, wide excision is associated with lower recurrence rates and may be curative.5,7 Sites with higher recurrence rates include perianal, vulvar, and inferior breast. Surgical outcomes can improve with prior medical treatment, especially with biologic treatment.5
Laser Therapy
There are no standardized treatment protocols in laser and photodynamic therapy (PDT), making it hard to compare treatment modalities. Examples of laser therapies include long-pulsed neodymium-doped yttrium aluminum garnet laser (Nd:YAG), CO2 laser, and Alexandrite laser. Studies have shown clinical improvement, with decreased inflammation, usefulness in de-roofing and destruction of lesions, and treatment of scars.5 PDT uses photosensitizers (5-aminolevulinic acid [ALA], methyl aminolevulinate [MAL], and methylene blue [MB]) applied topically or intralesionally with various light sources, (red light [635 nm]), blue light, intense pulsed light (630 nm), and laser diode [400 nm]). It has been associated with good clinical outcomes with intralesional PDT and axillary area showing better response rates.5 Photobiomodulation is also an option and has been shown in in-vitro HS models to promote wound healing, vasodilation, angiogenesis, and pain and inflammation relief.5
Biologic Treatments
As HS is a complex inflammatory disease, several biologics have been developed targeting several different molecules involved in the pathogenesis of HS.
Anti-TNF-α. Adalimumab (ADA) is a monoclonal antibody (mAb) and the only biologic approved for the treatment of moderate to severe HS by the FDA and European Medicine Agency. In the PIONEER I and II trials, use of ADA led to high HiSCR, fewer flares, and increased quality of life5. Infliximab (IFX), another mAb, has similar efficacy.5 Certolizumab pegol (CZP) is a fragment of IgG that binds to TNF-α. It may be useful in patients with moderate HS who are refractory to other biologic treatments or in patients who are pregnant, as CZP does not cross the placenta5. Etanercept competitively binds TNF-α receptors, and only 1 RCT has been done so far with no significant improvement in outcomes5. For golimumab (mAb), only 2 case studies have been done, with only 1 patient successfully treated.5
Anti-17. Secukinumab (SEC) is a mAb that helps patients achieve HiSCR with a potential association with the development or worsening of inflammatory bowel disease, though 1 study found no increased risk.5 Brodalumab (BRO) (mAb) blocks several isoforms of IL-17. Studies have shown rapid reduction of skin lesions and sinus tracts, with decreased recurrence with higher weekly doses.5 Bimekizumab (mAb) has recently shown efficacy in reducing disease severity. Currently, there is little evidence of the efficacy of ixekizumab (mAb) in the treatment of HS.5 CJM112, a new mAb, has had good response rates. It decreases the number of inflammatory lesions seen in HS.5
Anti-IL-12/23. UST is a mAb that binds to both IL-12 and IL-23. Response rates have been shown to be variable, especially in relation to doses, but patients who do respond often showed marked or moderate improvement with increased HiSCR.5
Anti-IL-23. Guselkumab (GUS) is a monoclonal antibody with variable results.5 Risankizumab (mAb) has shown some clinical response in a few studies5. Tidrakizumab (mAb) was studied in 1 case series that reported good clinical response and improvement in quality of life.5
Anti-IL-1. Anakinra competitively inhibits the IL-1 receptor. Its efficacy is unclear, with conflicting results. In studies that show significant decrease in disease severity and increased quality of life, the differences vs placebo disappear after a period of time or disease rebounds.5 MABp1 is a mAb that has been used in patients who failed or who had a contraindication to ADA, with most patients achieving HiSCR and maintaining efficacy after several months. Ultrasound also showed decreased angiogenesis and lesion depth7. Bermekimab (BER) (mAb) increases HiSCR in moderate to severe HS, including in patients with refractory disease or failure with anti-TNF agents.5Canakinumab (mAb) has unclear efficacy, with both positive and negative results.5
Other biologics. Apremilast (APR) is a phosphodiesterase-4 (PDE-4) inhibitor also with conflicting results.5 One study found decreased disease severity scores with persistence of treatment response, while another study found no statistically significant changes in inflammatory markers in treated vs placebo skin lesions.5 IFX-1 (mAb) binds to C5a that has shown good efficacy, achieving HiSCR in around 50% or more of patients in various studies.5 Tofacitinib is a JAK inhibitor that has been successfully used in severe HS resistant to other biologic treatments, with few adverse effects.5
Because multiple immunologic pathways are involved in HS, use of multiple different biologics may result in better responses than ADA monotherapy, though more research is needed.7
New Treatments
As the pathogenesis of HS is better understood, the number and efficacy of drugs for the treatment of HS will increase. However, the cytokine cascade of HS is complex and HS is very heterogeneous with various clinical presentations and different cytokine profiles. Early treatment is essential, so it is important to determine disease biomarkers and identify factors of poor prognosis and early disease progression.5
Topical Treatments
An in vitro study of a topical formulation of clindamycin and rifampin in nano-lipid carriers found that clindamycin and rifampicin accumulated in hair follicles, suggesting that lipid carriers could increase effectiveness of topical treatments.5
Phase II, open-label studies are investigating topical gentian violet, topical LTX-109, and topical ruxolitinib cream. Gentian violet has antiseptic and wound healing properties, LTX-109 has antimicrobial, anti-inflammatory, and antibiofilm effects, and ruxolitinib is a JAK1/JAK2 inhibitor.5
Hormonal Treatments
A phase III RCT is examining the efficacy or metformin with doxycycline compared with doxycycline monotherapy (new perspectives). For laser therapies, an open-label study is evaluating the use of Alexandrite laser hair removal in patients with HS in the axilla and groin.
Biologic Treatments
Several of the biologic HS treatments mentioned above require more trials establishing consistent efficacy. There are also many new drugs under investigation for their safety and efficacy (including optimal dosages) in the treatment of HS, listed below.
- SEC (mAb)5
- Bimekizumab (mAb)5
- GUS (mAb)5
- Risankizumab (mAb)5
- Avacopan (C5a receptor antagonist) and the role of C5a receptors in general in HS5
- JAK inhibitors, including INCB0547075
- Iscalimab (mAb that blocks the CD40-CD154 costimulatory pathway)5
- Spesolimab, ismidolimab (mAb against the IL-36 receptor)5
- LY 3041658 (mAb against chemokines that bind to CXCR1 or 2 receptors)5
- LYS 006 (leukotriene A4 hydrolase inhibitor)5
- KT-474 (degrades the IL-1 receptor-associated kinase 4 (IRAK4))5
- PF-06650833 (IRAK4 inhibitor)5
- PF-06650833 (tyrosine kinase 2 (TYK2) inhibitor)5
- PF-06700841 (TYK2/JAK1 inhibitor that prevents IL-12 and IL-23 signaling)5
- CSL324 (mAb against the granulocyte-colony-stimulating factor (G-CSF) receptor)7
Conclusions
HS is a chronic inflammatory skin condition that presents with painful lesions, abscesses, sinus tracts, and fistulas found in the axillary, inguinal, and genital regions. Its prevalence ranges between 0.05% and 4.1% and it has a significant impact on the health and professional/social lives of patients. There are several treatments available that target various molecules in the inflammatory pathways in HS both locally and systemically, including antibiotics, anti-inflammatories, hormonal treatments, and biologics, as well as lifestyle changes, surgery, and laser therapy. Many new treatments are also under investigation, widening the reach of treatment and its efficacy.
References
- Ingram J. The epidemiology of hidradenitis suppurativa. Br J Dermatol. 2020;183(6):990-998. doi:10.1111/bjd.19435
- Liy-Wong C, Pope E, Lara-Corrales I. Hidradenitis suppurativa in the pediatric population. J Am Acad Dermatol. 2015;73(5 suppl 1):S36-S41. doi:10.1016/j.jaad.2015.07.051
- Jemec GBE, Kimball AB. Hidradenitis suppurativa: epidemiology and scope of the problem. J Am Acad Dermatol. 2015;73(5 suppl 1):S4-S7. doi:10.1016/j.jaad.2015.07.052
- Sabat R, Jemec GBE, Matusiak Ł, et al. Hidradenitis suppurativa. Nat Rev Dis Primers. 2020;6(1):18. doi:10.1038/s41572-020-0149-1
- Amat-Samaranch V, Agut-Busquet E, Vilarrasa E, Puig L. New perspectives on the treatment of hidradenitis suppurativa. Ther Adv Chronic Dis. 2021;20406223211055920. doi:10.1177/20406223211055920
- van Straalen KR, Schneider-Burrus S, Prens EP. Current and future treatment of hidradenitis suppurativa. Br J Dermatol. 2020;183(6):e178-187. doi:10.1111/bjd.16768
- Goldburg SR, Strober BE, Payette MJ. Hidradenitis suppurativa: Current and emerging treatments. J Am Acad Dermatol. 2020;82(5):1061-1082. doi:10.1016/j.jaad.2019.08.089

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.