- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
News
Article
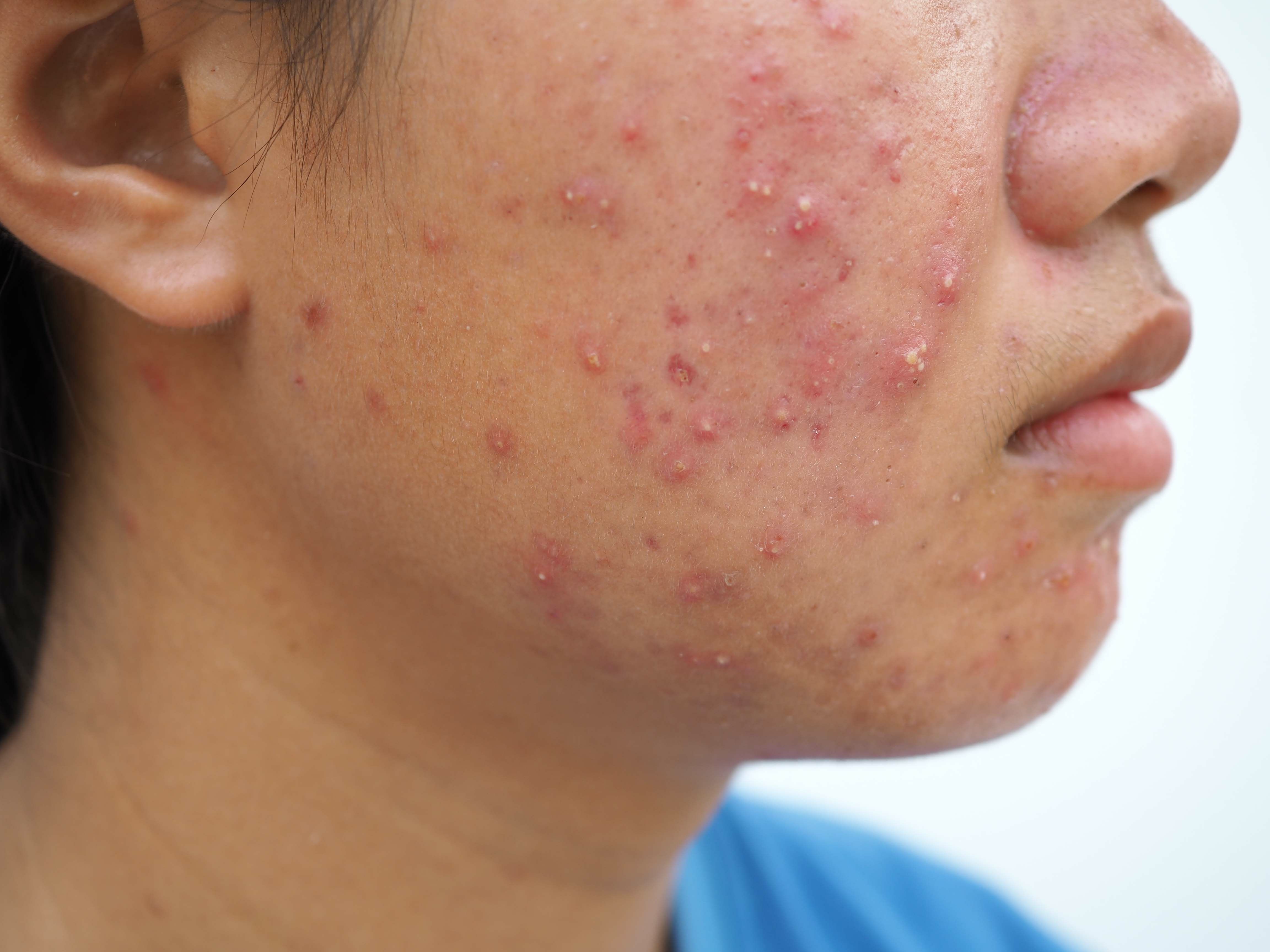
IDP-126 Gel Reduces Inflammatory and Noninflammatory Acne Lesions By 70%
Author(s):
Half of study participants achieved treatment success vs less than one-fourth with vehicle.
A recent poster presentation from Maui Derm NP & PA Fall in Asheville, North Carolina, examined the safety and efficacy of IDP-126 gel (clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide (BPO) 3.1%) as the first triple-combination, fixed-dose topical acne product in development that addresses the major pathophysiological abnormalities in patients with acne. According to Stein Gold et al, combining an antibiotic, antibacterial agent, and retinoid in a single formulation has the potential to provide greater efficacy than a single or double treatment, while also potentially reducing antibiotic resistance.1
The study authors analyzed one phase 2 study (n=741; NCT03170388) and 2 phase 3 studies (n=183; n=180; NCT04214639; NCT04214652), which were all double-blind, randomized, 12-week studies and enrolled patients aged ≥9 years with moderate-to-severe acne. In the phase 2 study, patients were randomized 1:1:1:1:1 to receive IDP-126 gel (n=147), BPP/adapalene gel (n=150), clindamycin phosphate/BPO gel (n=146), clindamycin phosphate/adapalene gel (n=150), or vehicle gel (n=148). In the first phase 3 study, patients were randomized 2:1 to receive IDP-126 gel (n=122) or vehicle gel (n=61). In the second phase 3 study, patients were randomized 2:1 to receive IDP-126 gel (n=120) or vehicle gel (n=60).
End points included patients achieving ≥2-grade reduction from baseline in Evaluator’s Global Severity Score (EGSS) and clear/almost clear skin (treatment success) and least-squares mean percent change from baseline in inflammatory and noninflammatory lesion counts. Treatment-emergent adverse events (TEAEs) were also assessed.
In the phase 2 trial, 52.5% of patients receiving IDP-126 gel achieved treatment success at week 12. In the first phase 3 trial, 49.6% of patients receiving IDP-126 gel achieved treatment success at week 12 compared to 24.9% of the vehicle gel group. Lastly, in the second phase 3 trial, 50.5% of patients receiving IDP-126 gel achieved treatment success at week 12 compared to 20.5% of the vehicle gel group.
Regarding mean percent changes from baseline in lesion counts at week 12, in the phase 2 study, 76.4% of patients receiving IDP-126 gel had a mean percent change from baseline in inflammatory lesions. In the first phase 3 study, 75.7% of patients receiving IDP-126 gel had a mean percent change from baseline in inflammatory lesions compared to 59.6% of the vehicle gel group. In the second phase 3 study, 80.1% of patients receiving IDP-126 gel had a mean percent change from baseline in inflammatory lesions compared to 56.2%.
For noninflammatory lesions, in the phase 2 study, 71.0% of patients receiving IDP-126 gel had a mean percentage change from baseline. In the first phase 3 study, 72.7% of patients receiving IDP-126 gel had a mean percent change from baseline compared to 47.6% of the vehicle gel group. In the second phase 3 study, 73.3% of patients receiving IDP-126 gel had a mean percent change from baseline compared to 49.0% of the vehicle gel group. Overall, “IDP-126 resulted in over 70% reductions of inflammatory and noninflammatory lesions at week 12, significantly greater than vehicle and each dyad combination.”
In 4 patient cases included in the poster presentation, 3 out of the 4 patients had over a 90% decrease in inflammatory lesions and one patient had an 87% decrease in inflammatory lesions.
Overall, IDP-126 was well tolerated across all 3 studies. Less than 4% of patients discontinued studies/treatment due to adverse events. Most reported TEAEs were mild or moderate.
Stein Gold et al concluded that “The innovative fixed-dose, triple-combination IDP-126 gel was efficacious and well-tolerated in 3 clinical studies including children, adolescents, and adults with moderate-to-severe acne. To our knowledge, observed acne improvements in these studies with IDP-126 were greater than any FDA-approved topical acne treatment, though patient populations may differ across studies.”
Reference
- Stein Gold L, Kircik L, Tanghetti E, et al. Efficacy and safety of a fixed-dose clindamycin phosphate 1.2%/adapalene 0.15%/benzoyl peroxide 3.1% gel for moderate-to-severe acne: randomized phase 2 and phase 3 studies of the first triple-combination drug. Presented at: Maui Derm NP & PA Fall; September 27-30, 2023; Asheville, NC.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.