- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Immunotherapy Helps Manage Skin Disease in Patients with Cancer
Author(s):
There are an increasing number of novel immunotherapies available for patients with skin cancer, however, their numerous adverse events must be addressed.
Research has led to the continued development and FDA approval of several immunotherapeutic medications proven to be successful in an increasing number of diseases in medicine, including dermatology. Although found to be effective, these increasingly popular targeted therapies and immune checkpoint inhibitors are also associated with cutaneous toxicities that every clinician should be aware of and prepared to appropriately address when prescribing these medications.
Novel cancer therapeutics include targeted inhibitors such as oral small molecule tyrosine kinase inhibitors (TKI), and intravenous monoclonal antibodies targeted to a specific molecular mutation or combination of mutations in a tumor. According to a Anisha B. Patel, MD, FAAD, these medications are being used more frequently in combination therapies such as BRAF and MEK inhibitors for melanoma, EGFR and MEK inhibitors for colorectal cancer, multikinase and mTOR inhibitors for renal cell cancer, and EGFR and mTOR inhibitors for breast cancer.
Anisha B. Patel, MD, FAAD

“Targeted inhibitors have closely linked protein signals and therefore similar overlapping toxicities between the different therapies. However, this makes the toxicity profiles even more difficult to parse out at times,” said Patel, an associate professor in the Department of Dermatology, Division of Internal Medicine, at The University of Texas MD Anderson Cancer Center and McGovern Medical School in Houston. Patel gave a presentation on this topic at the American Academy of Dermatology 2021 Virtual Meeting Experience in April.
The novel checkpoint inhibitors are part of a new category of immune therapy most frequently used in cancer treatments today. According to Patel, these agents have a less specific action than targeted inhibitors and work by blocking the inhibition of host T cell immune response to the tumor. In the anti–CTLA-4 pathway, CTLA-4 inhibition causes the host immune response to be appropriately activated to the foreign body tumor. Programmed cell death 1 (PD-1) and its ligand are thought to be more specific to the tumor cells, and therefore it is thought that their blockade should have reduced off-target adverse effects. According to Patel, this can be seen in the lower incidence of cutaneous toxicities compared with anti–CTLA-4 therapies.
Similar to the CTLA-4 inhibitors ipilimumab (Yervoy; Bristol Myers Squibb) and tremelimumab (an investigational checkpoint inhibitor; Astrazenca), anti–PD-1 inhibitors such as nivolumab (Opdivo; Bristol Myers Squibb), pembrolizumab (Keytruda; Merck), and cemiplimab (Libtayo; Sanofi Genzyme/Regeneron Pharmaceuticals); and PD-L1 inhibitors including atezolizumab (Tecentriq; Genentech), avelumab (Bavencio; EMD Serono), and durvalumab (Imfinzi; AstraZeneca) allow host T-cell immune response to the tumor cells. Numerous medications have been FDA approved in the checkpoint inhibitor category, Patel said, and these drugs are being used more frequently in combination with increased incidence and severity of adverse events.
“For the practicing dermatologist, it’s important to put this information in perspective because we are taught that antibiotics have historically been the No. 1 cause of drug rashes, but even at their highest incidence they don’t even reach 10% incidence for monotherapy. In contrast, checkpoint inhibitors and targeted inhibitors start with [approximately] a 10% incidence rate,” Patel said.
Depending on the specific drug and whether it is used as a monotherapy or as combination therapy, Patel said that the incidence rates of cutaneous toxicities can be as high as 60% in checkpoint inhibitors and 100% when EGFR inhibitors are used with MEK inhibitors or checkpoint inhibitor therapy.
Among the EGFR inhibitors, the TKI inhibitors have the lowest incidence at approximately 10%. However, Patel said, there is a sharp increase when these agents are combined with MEK or checkpoint inhibitors, invariably resulting in a drug rash. According to Patel, even more significant than the rash incidence is the rate that these drugs affect the patient’s ability to remain on lifesaving or life-prolonging cancer therapy.
“Whereas the EGFR TKI inhibitors have a low impact rate of cutaneous toxicities, checkpoint inhibitors have [approximately] a 10% incidence rate that can cause a dose delay or discontinuation,” Patel said. “However, the monoclonal antibodies used alone or in combination have a 50% to 100% chance of rashes severe enough to cause a dose reduction, delay, or discontinuation. Dermatologists have the potential to play an important role in managing toxicities and keeping patients on their needed cancer therapies.”
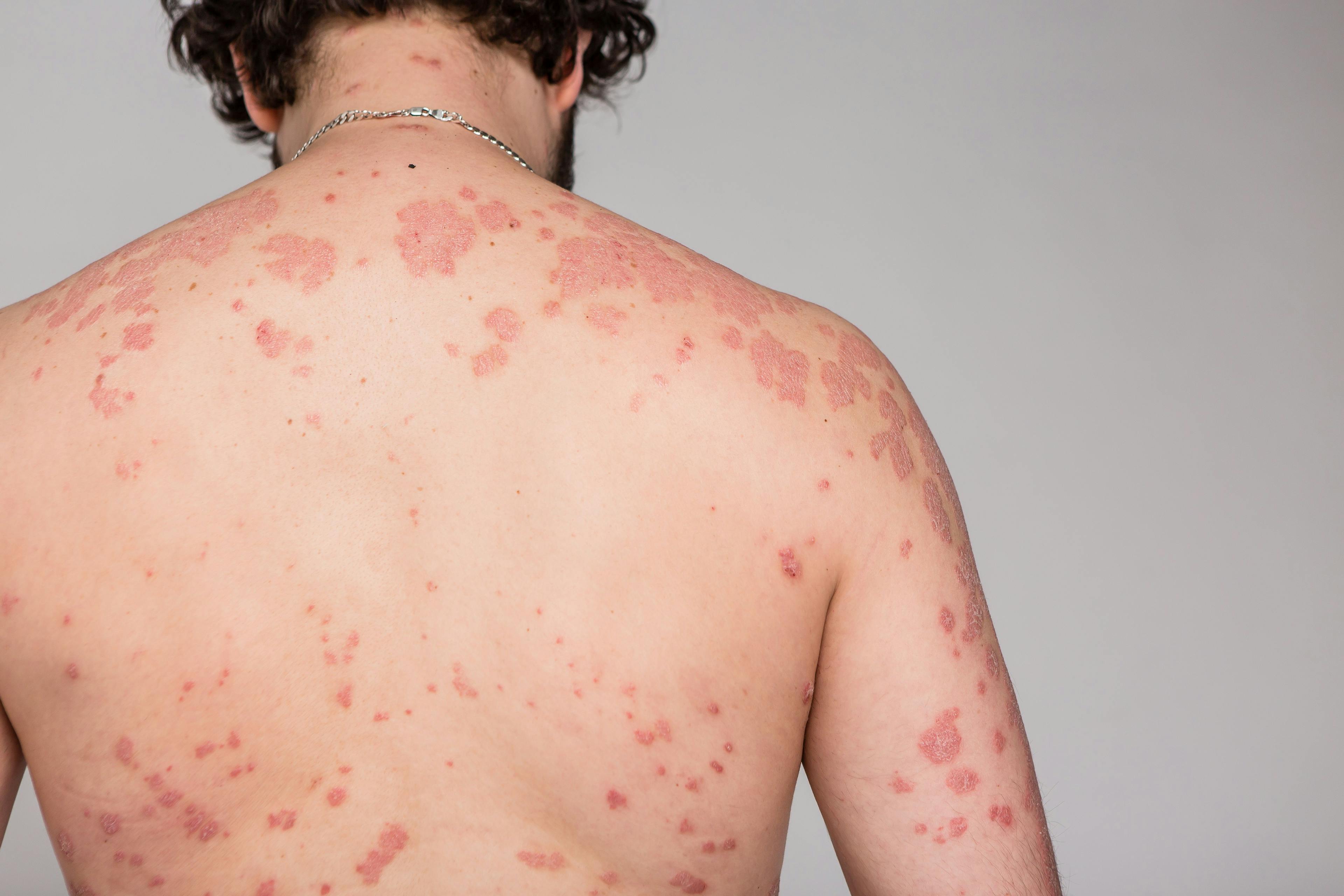
Fortunately, the rashes and adverse events that are typically encountered with targeted inhibitor therapy, including—but not limited to—acneiform eruptions, paronychia, keratotic neoplasms, keratosis pilaris, erythema nodosum, phototoxicity, hand foot skin reaction, eczema, periorbital edema, alopecia, xerosis, and onycholysis, are not new to dermatologists and can be addressed appropriately in a timely fashion.
According to Patel, P13K, FGFR, and Nectin-4 inhibitors and their cutaneous toxicities are having a particular impact on cancer treatment. For P13K inhibitors, the incidence rates across the trials are significant and up to almost 50%. Although the all-grade incidence of cutaneous adverse events is up to 65% in FGFR inhibitors and up to 7% for severe grade cutaneous adverse events, severe grade rashes occur in up to 10% of patients on Nectin-4 inhibitors.
Some of the most common cutaneous adverse events that occur in association with checkpoint inhibitors include morbilliform drug eruptions, eczema, psoriasis, lichenoid eruptions, vitiligo, lupus erythematosus, bullous pemphigoid, and nonmelanoma skin cancer (NMSC). These rashes appear after 1 to 2 cycles of treatment initiation, according to Patel, with bullous pemphigoid and neoplastic changes having a longer time to onset.
“The histologic findings are typical of a non–drug-associated skin disease, and the eruptions are managed with traditional therapies of topical steroids, oral antihistamines, and light therapy for the most part,” Patel said.
Acneiform eruptions are typically associated with the EGFR, MEK, mTOR inhibitors, and, less frequently, checkpoint inhibitor therapy. The etiology is different than typical acne as follicular plugging is not the issue, Patel said. Instead, follicular instability and the resulting inflammatory reaction lead to the rash. Here, it is recommended that patients start with a bland emollient and low potency topical steroids for flares. In addition, therapies with alcohol bases or those that dry out the skin should be avoided because they can exacerbate the dryness that these patients are prone to and cause painful microfissures. For more severe rashes, a medium potency topical steroid as well as oral doxycycline can be added to the treatment regimen.
The same drugs that cause acneiform eruptions are also associated with paronychia, according to Patel, where time to onset is typically later than acneiform eruptions and can be quite prolonged. Conservative therapy can include mechanical means to treat or prevent ingrown nails, superinfection prevention, and topical anti-inflammatories.
“It is not uncommon that conservative therapy can be very slow going and, as such, it is important for us to remember that more aggressive interventions, including surgical intervention, are always an option. If all else fails, discussing with oncology and recommending oral steroids or dose reduction is the last resort,” Patel said.
New cutaneous adverse events are constantly being reported in association with different drugs, but the majority are treated with standard dermatology practices and present the opportunity for dermatologists to be an important part of the cancer team.
“We are having increasing management challenges with combination therapies necessitating creative therapies, prospective trials of biologics, and new drug development where we have no other options,” Patel said. “Dermatologists are an important part of the cancer team, and communication with our colleagues and patients is critical to understanding their needs and keeping our patients on the most effective cancer therapy possible.”
Disclosures: Patel reported no relevant disclosures.
Reference:
1. Patel A. Cutaneous toxicities to targeted and immunotherapies: update and management. American Academy of Dermatology 2021 Virtual Meeting Experience; April 23-25, 2021; virtual. Accessed June 8, 2021

Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.