- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Ongoing Study Evaluates Combination Therapy for Androgenetic Alopecia
Author(s):
An ongoing study is evaluating the use of multimodal aesthetics protocol in treating patients with hair loss.
A current, ongoing study1 is examining multimodal treatment for hair loss, particularly in patients with the most common form of hair loss among both men and women: androgenetic alopecia (AGA).
Aisylu/AdobeStock
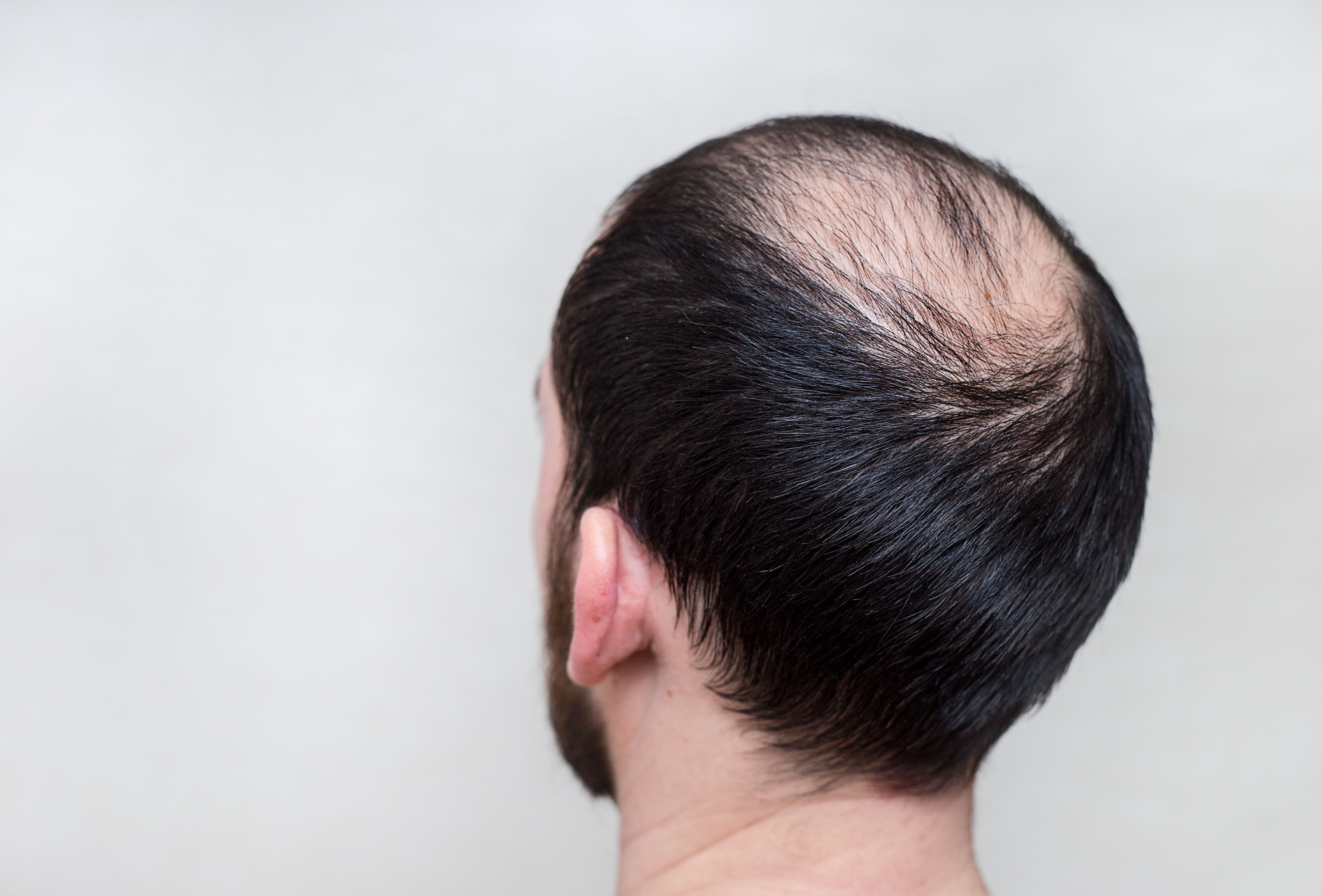
Revian Inc., a medical technology company specializing in aesthetics and hair/skin rejuvenation, announced its involvement in the study via a recent press release.1 Revian’s RED Hair Regrowth System is currently the first and only all-LED hair loss device cleared by the US Food and Drug Administration (FDA) for hair regrowth.
The device is a wireless cap that uses dual wavelength LED technology and is controlled by a mobile app. The system uses a dual-combination of 2 LED wavelengths to “stimulate the production and release of nitric oxide, proven to increase local blood flow, reduce inflammation, and inhibit DHT production which provides the right environment for new hair growth,” according to the press release.
The study, which began in July 2022, is being conducted by Spectrum Advanced Aesthetics in 2 phases. Participants included male and female patients ages 18 to 65 years old with hair loss and without other underlying conditions. Researchers examined all participants using a baseline scalp sonogram, hair loss classification, hair loss rating, and medical grade imaging prior to their enrollment in the study.
The first phase, the correction phase, uses in-clinic procedures and home-based treatments as combination therapy. Participants are treated with Hydrafacial Keravive scalp treatments, microneedling, and ultrasound in in-clinic settings. At home, they are instructed to use a combination of several treatments, including hair care products, nutraceuticals, and the Revian RED Hair Regrowth System.
During the second phase, the maintenance phase, participants are treated with 3 Hydrafacial Keravive scalp treatments every 4 weeks. They are also instructed to participate in quarterly in-clinic follow-up visits, and quarterly platelet-rich plasma (PRP) and microneedling treatments as recommended. They are also asked to continue their use of at-home treatments from the first phase.
Phase 1 is anticipated to conclude by the end of 2023, while more data is expected in early 2024. Within the first cohort of participants, researchers have already noted increased hair density, increased scalp blood flow, new terminal hair growth, and reduced scalp sensitivity.
"With the onset of the COVID-19 Pandemic, reported premature hair loss from men and women has increased substantially, along with a spike in online searches for hair loss causes, remedies, and treatments," said Selisha Abbas, Spectrum Advanced Aesthetics lead researcher, in the press release. "Revian Red's ease of use and best in class performance made it the clear choice for us to include as a critical part of the protocol."
Reference
- New study includes Revian red as part of multimodal treatment protocol for hair loss. PR Newswire: press release distribution, targeting, monitoring and marketing. May 11, 2023. Accessed May 12, 2023. https://www.prnewswire.com/news-releases/new-study-includes-revian-red-as-part-of-multimodal-treatment-protocol-for-hair-loss-301822771.html.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.