- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Oral Nicotinamide Not Beneficial in Preventing Skin Cancer in Organ Transplant Recipients
Author(s):
Transplant patients are at a higher risk of nonmelanoma carcinomas due to immunosuppressive therapies.
A 12-month, placebo-controlled trial found that oral nicotinamide (vitamin B3) therapy did not lead to lower numbers of keratinocyte cancers or actinic keratoses in immunosuppressed solid-organ transplant recipients.1
For the phase 3 Oral Nicotinamide to Reduce Actinic Cancer After Transplant (ONTRANS) trial, investigators randomly assigned 158 organ-transplant recipients who had been diagnosed with at least 2 keratinocyte cancers in 5 years to receive 500 mg of nicotinamide or placebo twice daily for 12 months (1:1 ratio). Dermatologists examined patients for skin lesions at 3-month intervals during that time. The primary endpoint was the number of new keratinocyte cancers during that time period; secondary endpoints added in the number of squamous cell and basal cell carcinomas during the intervention period, the number of actinic keratoses until 6 months after randomization, safety, and quality of life.
2702707195204/AdobeStock
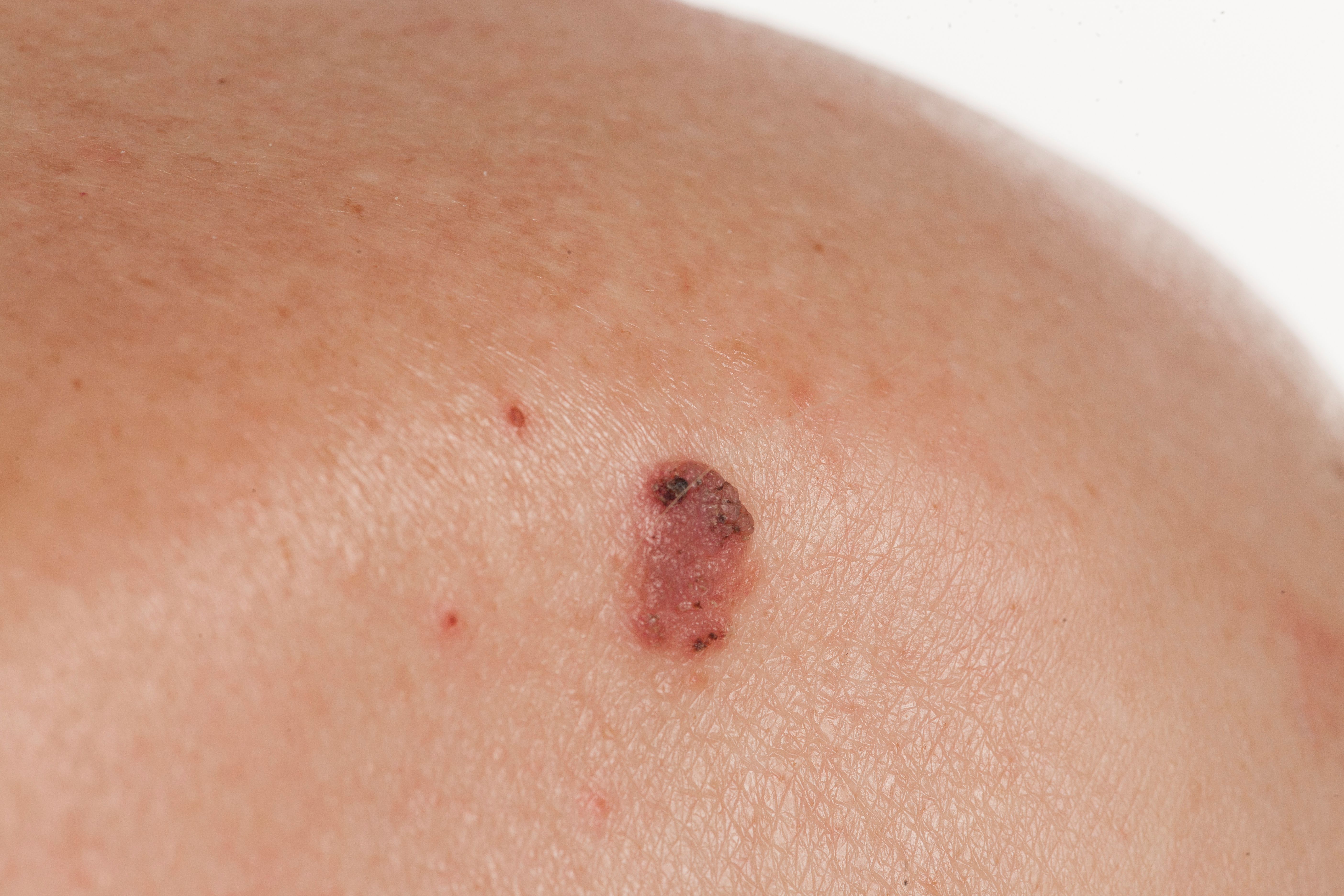
Study investigators at the University of Sydney and Westmead Hospital in New South Wales, Australia said their findings contradict previous findings of the Oral Nicotinamide to Reduce Actinic Cancer (ONTRAC) trial, which showed a 23% lower rate of skin cancers in patients on oral nicotinamide versus placebo. The study authors noted that the ONTRANS trial ended early due to low recruitment numbers (158 out of a planned 254 participants).
Nonmelanoma skin cancers are the most common malignancies in organ transplant recipients, and carcinomas tend to be more aggressive and have a much higher incidence of metastasizing compared to the general population.2 Nicotinamide has been shown to prevent nonmelanoma skin cancers in healthy, immunocompetent people, and has been widely used as a potential chemo preventive agent in the solid organ transplant patient population.
References
1. Allen NC, Martin AJ, Snaidr VA, et al. Nicotinamide for Skin-Cancer Chemoprevention in Transplant Recipients. N Engl J Med. 2023 Mar 2;388(9):804-812. doi: 10.1056/NEJMoa2203086. PMID: 36856616.
2. Rudež LK, Šklebar T, Čeović R. Nonmelanoma Skin Cancer in a Heart Transplant Patient: A Case Report and Review of the Literature. Acta Clin Croat. 2022 Mar;61(1):157-165. doi: 10.20471/acc.2022.61.01.21. PMID: 36398077; PMCID: PMC9616031.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.