- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Article
Positive phase 3 data for cantharidin topical solution announced
Author(s):
Verrica released positive data from its post-hoc pooled analysis of pivotal phase 3 trials investigating the safety and efficacy of VP-102 for the treatment of molluscum contagiosum.
Dr. Eichenfield

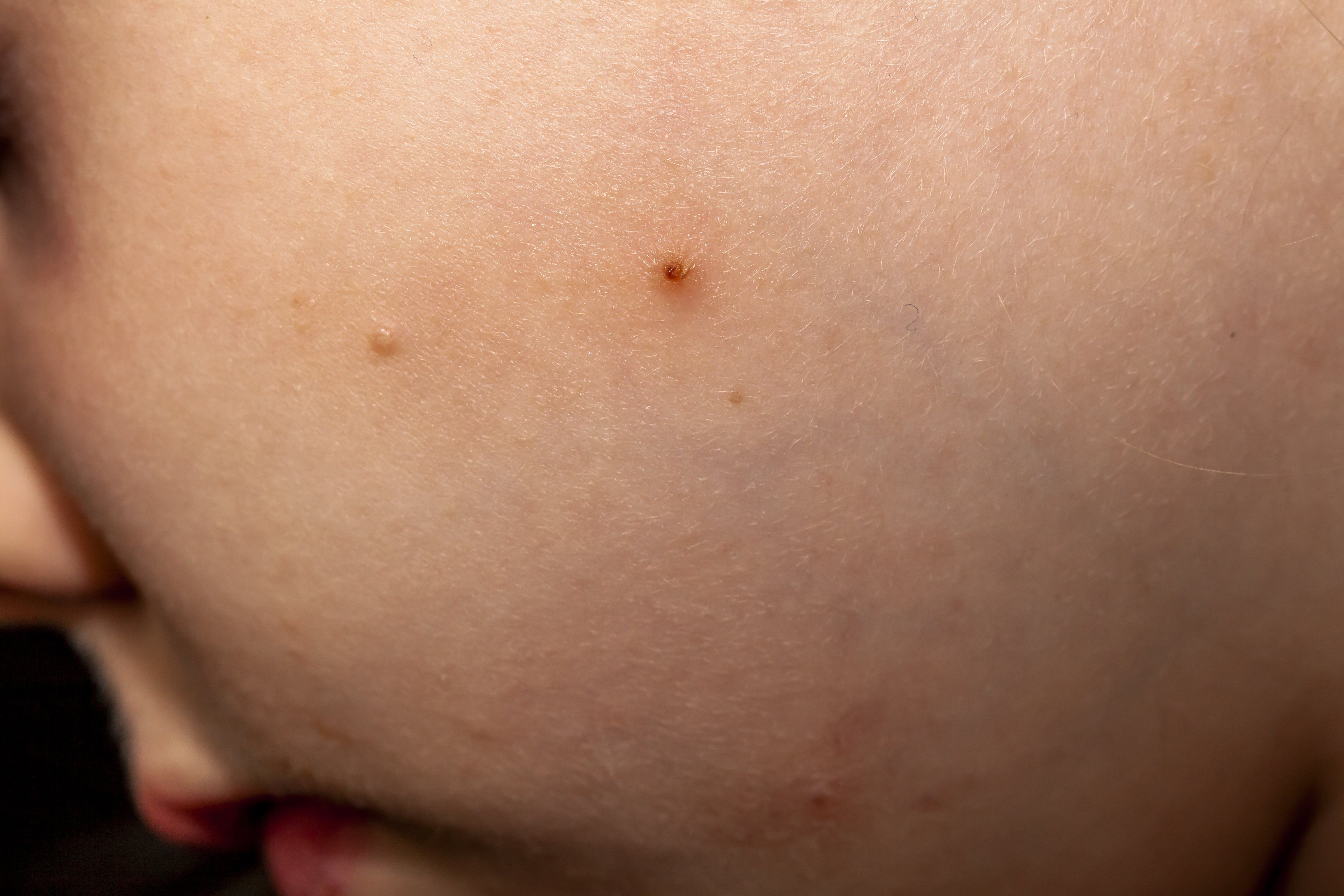
VP-102 (cantharidin 0.7% topical solution) may offer significantly higher, widespread clearance of lesions for patients with molluscum contagiosum (molluscum).
The company released positive data from its post-hoc pooled analysis of pivotal phase 3 trials on Jan. 18 at the 17th Annual Winter Clinical Dermatology Conference in Kohala Coast, Hawaii. The trials investigated the safety and efficacy of VP-102 for the treatment of the viral skin infection across all areas of the body.
“The results of this study are important, as they suggest that this investigational molluscum treatment can potentially bring about complete clearance, regardless of where on the body the lesion is located,” says Lawrence Eichenfield, M.D., chief of pediatric and adolescent dermatology, Rady Children’s Hospital, San Diego, and principal investigator of the VP-102 phase 3 trial.
During the trial, researchers examined participants with lesions across six body regions: head/neck (n=77 VP-102, n=53 vehicle), chest/abdomen (n=142, 118), back/buttocks (n=117, 91), groin (n=28, 25), upper extremities (n=179, 131) and lower extremities (n=186, 141). After determining a baseline for each patient, investigators measured clearance in each location during follow-up to determine efficacy of treatment versus vehicle.
RELATED: Wart treatment clinical trials hold promise
The results showed individuals who received VP-102 experienced a statistically higher percentage of complete lesion clearance by day 84 across all body areas compared to vehicle. Most notably, patients receiving VP-102 experienced the greatest percentage of clearance on what is considered “sensitive” body areas, including the head and neck (81%) as well as the groin (85.7%).
“If approved, VP-102 may potentially be an important option for physicians, as they can have the option to treat even the most sensitive areas, including the groin, head and neck,” says Dr. Eichenfield.
Treatment emergent adverse events (TEAEs) included application site vesicles, pain, itchy skin, scabbing, erythema, dryness, discoloration and edema, and appeared to be similar across all body regions.
If approved, VP-102 would be the first FDA-approved treatment for molluscum.
READ MORE: Verrica develops solution for common warts
References:
Verrica Pharmaceuticals Announces Presentation of Positive Data from New Pooled Analysis of Phase 3 CAMP Trials of VP-102. Yahoo! Finance. https://finance.yahoo.com/news/verrica-pharmaceuticals-announces-presentation-positive-150010610.html. Published January 18, 2020. Accessed January 20, 2020.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.











