- Acne
- Actinic Keratosis
- Aesthetics
- Alopecia
- Atopic Dermatitis
- Buy-and-Bill
- COVID-19
- Case-Based Roundtable
- Chronic Hand Eczema
- Chronic Spontaneous Urticaria
- Drug Watch
- Eczema
- General Dermatology
- Hidradenitis Suppurativa
- Melasma
- NP and PA
- Pediatric Dermatology
- Pigmentary Disorders
- Practice Management
- Precision Medicine and Biologics
- Prurigo Nodularis
- Psoriasis
- Psoriatic Arthritis
- Rare Disease
- Rosacea
- Skin Cancer
- Vitiligo
- Wound Care
Publication
Article
Dermatology Times
Wart treatment clinical trials hold promise
Author(s):
New drugs in clinical trials for the treatment of warts and molluscum contagiosum could improve on currently available therapies, which are limited, used off-label and often cause tissue damage.
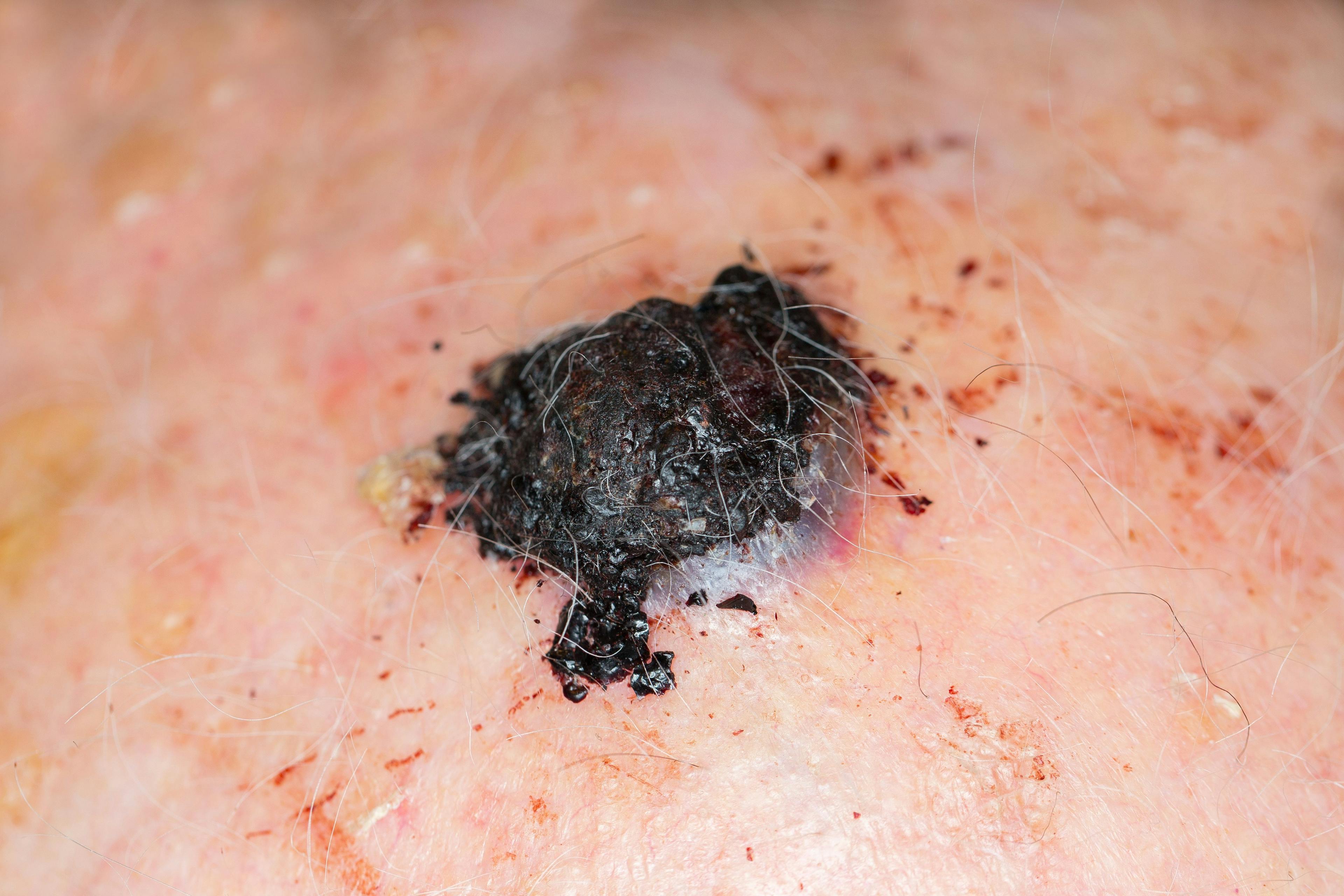
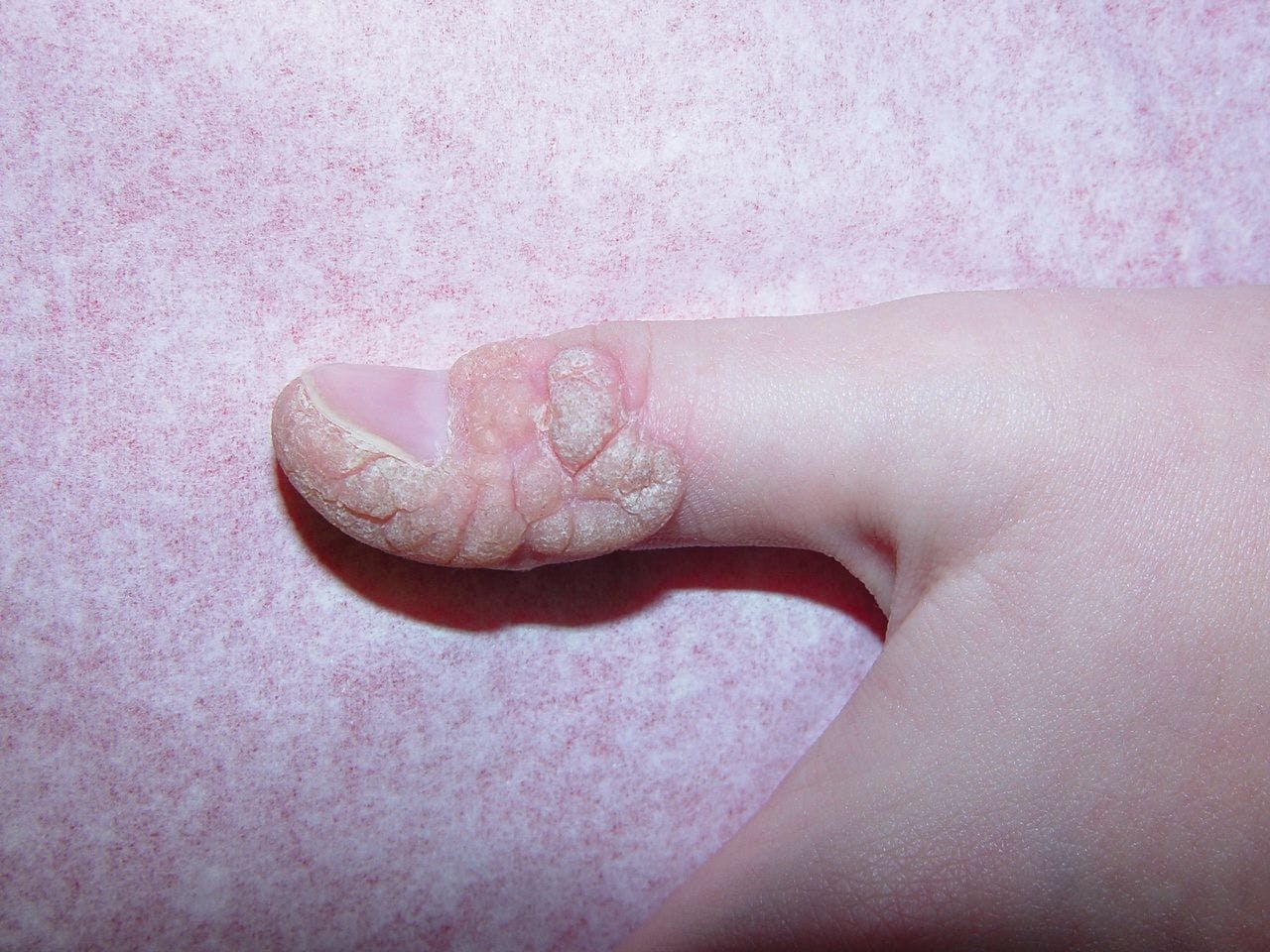
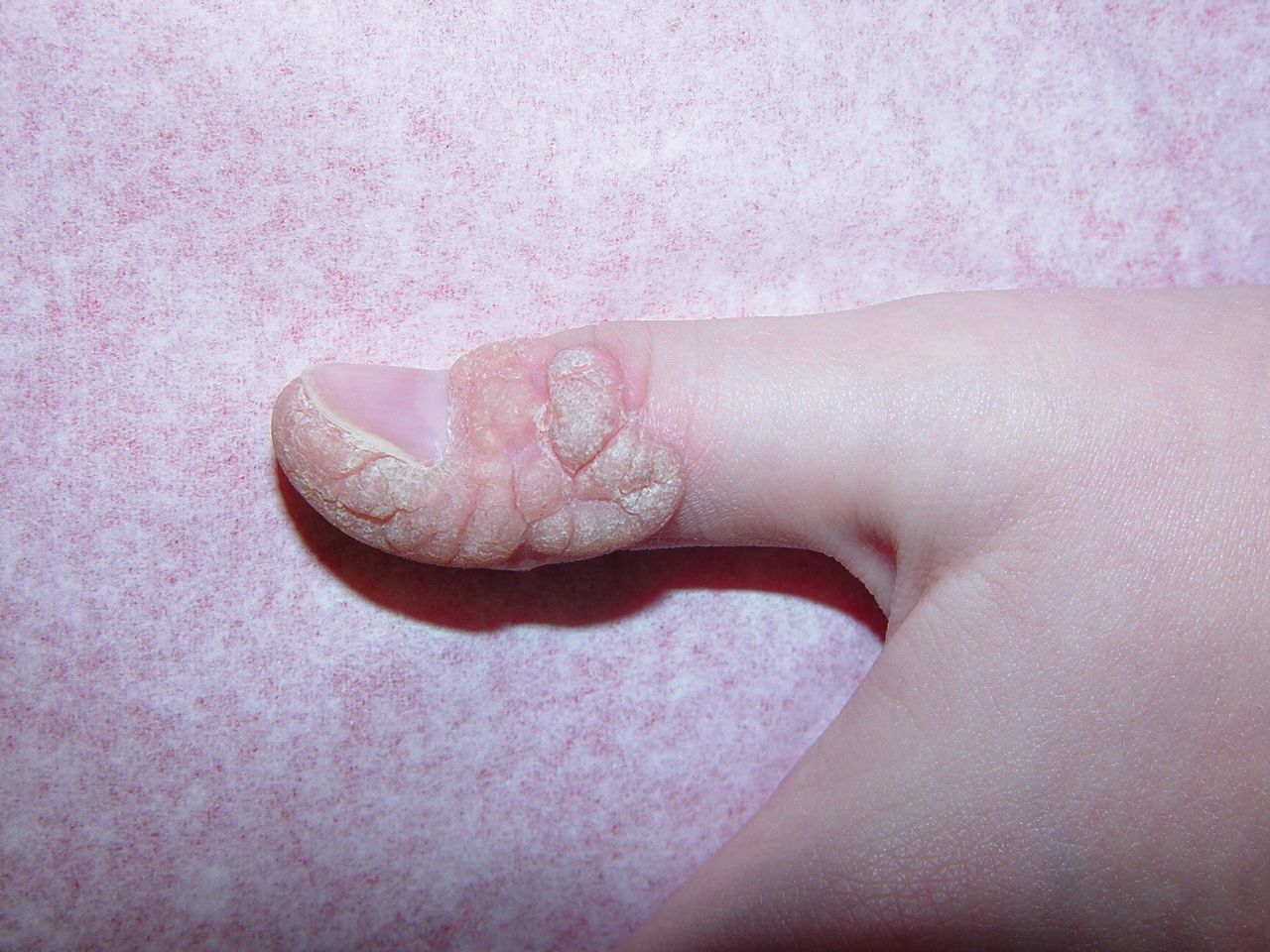
Koebnerization of wart after combination Cantharidin. (Photo courtesy of Elaine Siegfried, M.D.)
There are several drugs being studied for the viral skin infections that cause warts and molluscum contagiosum1, which may provide more effective treatment options than what is currently available.
While these conditions are self-limiting, and will eventually resolve themselves, untreated warts could last two years, and molluscum contagiosum could last one year; although, both conditions could last much longer.
Current treatments have limited effectiveness and are used off label, which can cause additional discomfort through tissue damage. Unless the conditions affect pressure points on the skin and cause discomfort, the biggest concerns for most patients are social and cosmetic. As a result, the best approach may be to let the condition run its course, some experts say.
TREATMENTS IN DEVELOPMENT
Clinical trials are primarily examining patients with molluscum contagiosum, which has no current treatment approved by the U.S. Food and Drug Administration, and in genital warts. Genital warts are the focus rather than common warts, because certain HPV strains that cause them are implicated in cancer and are less social acceptable, but also because efficacy may be easier to demonstrate in as the skin of genital warts is less heavily keratinised than common warts thus boosting the potential bioavailability of any topical treatments tested.
Novan is developing SB206 as a topical antiviral gel for the treatment of viral skin infections, with a current focus on the treatment of molluscum contagiosum, a contagious skin infection caused by the molluscipoxvirus, and external genital warts (EGW) caused by human papillomavirus (HPV). SB206 is a topical berdazimer sodium gel co-administered with hydrogel which promotes release of nitric oxide (NO), a free radical gas, which is endogenously produced in human cells. In high concentration, NO neutralizes many disease-causing microbes and has the potential to treat skin diseases caused by viruses. If approved, SB206 could be a prescription topical treatment for use at-home.
SB206 has entered phase 3 trials (NI-MC301, NI-MC302) in molluscum patients. Plans are to recruit 340 patients aged 6 months and older per trial; patients will receive SB206 12% once-daily or placebo in a 2:1 ratio.2,3
In a phase 2 dose-ranging trial 12% once-daily was the most effective dose with 42% (mITT, p<0.05) and 38% (ITT, p<0.05) complete clearance rates compared to 20% and 18% for vehicle, respectively.4,5
In a phase 2 dose-ranging clinical trial for the treatment of external genital warts, 33.3% of patients achieved complete clearance of baseline warts by week 12 when treated with SB206 12% once-daily, compared to only 4.3% of patients with vehicle once-daily (p=0.010).6
Paula Brown Stafford, chief development officer, Novan, says the treatment showed potential as a once-daily, at-home, safe and well tolerated topical therapy with high complete clearance rates and a treatment benefit as early as week two.
“If approved, [the treatment] would benefit a significant number of patients currently facing an inadequate treatment paradigm,” she says.
The drug which is closest to approval is Verrica Pharmaceuticals’s VP102, a topical application of cantharidin, which is naturally derived from the blister beetle, and has a long track record of treating cutaneous molluscum contagiosum and verrucae,7 reaching as far back as the ancient Egyptians. “Spanish fly” has been also used as an aphrodisiac, most notably the Marquis de Sade, with fatal consequences.
Although not approved by the United States Food and Drug Administration, cantharidin has been available through a variety of compounding sources without standardization of manufacturing, formulation, or method of application.
Both phase 2 and phase three trials have completed. Results from the two phase 3 trials were presented at the 2019 American Academy of Dermatology Meeting in March. A total of 528 patients aged at two years and older with molluscum were enrolled in the two trials (CAMP-1 and CAMP-2). After 12 weeks, 46% and 54% of subjects treated with VP-102 (topical solution of 0.7% cantharidin) every 21 days for up to four applications showed complete clearance of molluscum lesions compared with 18% and 13% of subjects in the placebo groups (p<0.0001).8
If approved, VP-102 will be a product applied by physicians, and likely to be used as an alternative to liquid nitrogen and other cryotherapy. The active ingredient cantharidin 0.7% is presented in single-use containers of a film formulating solution with a violet dye, so that the physician can see where it has been applied.
Ted White, President and Chief Executive Officer of Verrica says VP-102 has the potential to become the standard of care for molluscum.
“Complete clearance of molluscum lesions in a short amount of time is important to patients, especially parents of young children who are impacted by this highly contagious skin infection,” he says.
Veloce BioPharma LLC has tested its topical gel product VBP-245 in a small phase 2 trial at three sites in the United States,9 but the results have not yet been revealed, and Picato, an FDA-approved treatment for actinic keratosis is being tested in an early phase 1 trial in adults with molluscum,10 after case reports suggested it may be effective.11
So what should you offer patients with molluscum and warts now?
For common warts the go to office treatment is applying liquid nitrogen, although the equipment for this is expensive so some physicians may offer cryotherapies using fluorinated hydrocarbons instead, which is likely to be less effective than applying liquid nitrogen as they are not as cold.12 Over the counter, the traditional treatments are salicylic acid or cryotherapy based on fluorinated hydrocarbons.
For molluscum, options include liquid nitrogen in the office; topical treatment with podophyllotoxin cream (0.5%), salicylic acid, potassium hydroxide, tretinoin, cantharidin and imiquimod (T cell modifier); oral cimetidine; and even injections of interferon in immunocompromised individuals. However, physicians should take note that an inflamed lesion the inflamed lesion is often an indication of the beginning of the end - an expected variation in the evolution of immune response to the virus rather than bacterial superinfection.13
Elaine Siegfried, M.D., professor of dermatology and pediatrics at St. Louis University School of Medicine, St. Louis, Mo., says physicians often feel obliged to do something because children’s caregivers want them to do something.
Although the placebo effect of destructive treatments is likely to be strong, unless there is a good reason for treating, such as the site of the wart, it is often better to leave it or do something benign such as covering it with tape, which has been shown to have some effect,14 she advises.
“One of my favorites to use these days as a placebo is garlic, because garlic is cheap and readily available. The only downside of using garlic is that sometimes it’s toxic to tissue, but it’s not nearly as bad an erosion as cryotherapy or cantharidin,”15 Dr. Siegfried says.
Dr. Siegfried also sometimes suggests liquid zinc, although it can be hard to come by and hard to use as it usually requires zinc tablets to be crushed, and may cause stomach upsets.
For the paediatric dermatologist, the main issue of offering destructive treatments is that they can often be very uncomfortable for children while offering a low likelihood of success, she says.
“I’ve seen children who have been very negatively impacted by painful treatments,” Dr. Siegfried says. “It is very emotionally scarring.”
References:
1. Forbat E, Al-niaimi F, Ali FR. Molluscum Contagiosum: Review and Update on Management. Pediatr Dermatol. 2017;34(5):504-515.
2. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 200 Feb 29 - . Identifier NCT03927716, A Phase 3 Randomized Parallel Group Study Comparing the Efficacy & Safety of SB206 & Vehicle Gel in the Treatment of MC (B-SIMPLE1); 2019 Apr 25. Available from: https://clinicaltrials.gov/ct2/show/NCT03927716?term=sb206&cond=molluscum&rank=2
3. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 200 Feb 29 - . Identifier NCT03927703, A Phase 3 Randomized, Parallel Group Study Comparing the Efficacy & Safety of SB206 & Vehicle Gel in the Treatment of MC (B-SIMPLE2); 2019 Apr 25. Available from: https://clinicaltrials.gov/ct2/show/NCT03927703?term=sb206&cond=molluscum&rank=3
4. Novan Announces Full Top Line Results from Phase 2 Molluscum Contagiosum Clinical Trial and Decision to Move Forward with SB206 12% Once-Daily.; 2018 Dec 10. Available at: http://investors.novan.com/news-releases/news-release-details/novan-announces-full-top-line-results-phase-2-molluscum/. Accessed June 2019.
5. Maeda-Chubachi T, Hebert D, de Leon EN, Reams T, Messersmith E. Results of Phase 2 Study Evaluating the Efficacy and Safety of SB206, Topical Berdazimer Sodium Gel, in Subjects with Molluscum Contagiosum. Poster presented at: 2019 Society for Investigative Dermatology; May 8-11, 2019; Chicago, IL. Available from: http://novan.com/files/7315/5748/8389/SB206_SID_Molluscum_Poster_vFinal.pdf
6. Tyring SK, Rosen T, Berman B, Stasko N, Durham T, Maeda-chubachi T. A Phase 2 Controlled Study of SB206, a Topical Nitric Oxide-Releasing Drug for Extragenital Wart Treatment. J Drugs Dermatol. 2018;17(10):1100-1105.
7. Del rosso JQ, Kircik L. Topical Cantharidin in the Management of Molluscum Contagiosum: Preliminary Assessment of an Ether-free, Pharmaceutical-grade Formulation. J Clin Aesthet Dermatol. 2019;12(2):27-30.
8. Verrica Pharmaceuticals Presents Positive Results from Two Phase 3 Clinical Trials of VP-102 in Late-Breaking Oral Presentation at the 2019 American Academy of Dermatology Meeting.; 2019 Mar 2. Available at: https://investors.verrica.com/news-releases/news-release-details/verrica-pharmaceuticals-presents-positive-results-two-phase-3. Accessed June 2019.
9. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 200 Feb 29 - . Identifier NCT03077750, Study of VBP-245 in Pediatric Patients With Molluscum Contagiosum;2017 Mar 13. Available from: https://clinicaltrials.gov/ct2/show/NCT03077750?term=VBP-245&cond=molluscum&rank=1
10. ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 200 Feb 29 - . Identifier NCT03336372, Picato for the Treatment of Molluscum Contagiosum in Immunocompromised Patients; 2017 Nov 8. Available from: https://clinicaltrials.gov/ct2/show/NCT03336372?term=picato&cond=molluscum&rank=1
11. Del rosso JQ. Ingenol Mebutate Topical Gel A Status Report On Clinical Use Beyond Actinic Keratosis. J Clin Aesthet Dermatol. 2016;9(11 Suppl 1):S3-S11.
12. Burkhart C, Pchalek I. OTC cryotherapies not so cold. Dermatology Times. 2005;26(11):40. Available from: https://www.dermatologytimes.com/obstetrics-gynecology-womens-health/otc-cryotherapies-not-so-cold
13. Butala N, Siegfried E, Weissler A. Molluscum BOTE sign: a predictor of imminent resolution. Pediatrics. 2013;131(5):e1650-3.
14. Focht DR, Spicer C, Fairchok MP. The efficacy of duct tape vs cryotherapy in the treatment of verruca vulgaris (the common wart). Arch Pediatr Adolesc Med. 2002;156(10):971-4.
15. Schimmel J, Camarena-michel A, Hoyte C. Pediatric Cases Involving Chemical Burns to Garlic Used to Treat Warts. Dermatitis. 2019;30(1):80-82.
Newsletter
Like what you’re reading? Subscribe to Dermatology Times for weekly updates on therapies, innovations, and real-world practice tips.